If you’re interested in learning more about the 2021 Nobel prize in chemistry then our feature article goes into greater detail about what asymmetric organocatalysis is and why it won the prize and includes interview with both laureates.
In a rather unexpected move by the Nobel committee, this year’s prize in chemistry has been awarded to the two organic chemists who developed asymmetric organocatalysis more than two decades ago: Benjamin List from the Max-Planck-Institut für Kohlenforschung, Germany, and David MacMillan from Princeton University, US. Their work has shown that simple small organic molecules can match – and beat – enzymes and metal complexes at their own game: catalysing reactions to make chiral molecules.
Why did it win?
According to a 2015 estimate, catalysis contributes 35% of the world’s GDP. Catalysts have profoundly changed our understanding and use of chemistry, which is reflected in the fact that seven chemistry Nobel prizes have gone to discoveries in the field. The earliest went to Wilhelm Ostwald in 1909, who rationalised how a catalyst increases a chemical reaction’s rate but itself emerges unchanged from the reaction. Much more recently, in 2010, Richard Heck, Ei-ichi Negishi and Akira Suzuki were honoured for their discovery of palladium-catalysed cross couplings.
In catalysis, there is an emphasis on not only making reactions faster, but also doing asymmetric or enantioselective reactions – those that produce only one mirror image (enantiomer) of handed molecules. As certain biological molecules – amino acids and sugars – only occur as single enantiomers, our bodies have an inherent ability to distinguish between enantiomers. This means the same molecule can smell of orange or lemon depending on its handedness, and often only one enantiomer of a drug molecule has a beneficial effect, whereas the other does nothing or can even be harmful.
Until the start of the 21st century, most enantioselective catalysts were either enzymes or metal compounds. Enzymes usually can’t be made in the lab but must be isolated from biological sources. And while they work very well in the body, they don’t do so well under the conditions of synthetic chemistry, being inactivated by heat and solvents.
Transition metals, on the other hand, are excellent catalysts. But their very nature can make them problematic. Some metals are toxic to people or the environment, so they usually need to be removed from whatever organic compounds have been made with them. And some transition metals are so reactive that they need to be kept away from moisture or air to work, which makes it difficult and costly to use them on large scale.
This year’s laureates showed that even small, simple chiral compounds can catalyse complex reactions – just as well as, or in some cases even better than, enzymes or metals. Organocatalysts are often cheap and easy to produce, and have the potential to make synthetic routes greener.
Asymmetric organocatalysis is an ‘elegant tool’ that is ‘simpler than one could ever imagine’, said Nobel committee member Pernilla Wittung-Stafshede. Its discovery has allowed chemists to think of new and different way of putting together molecules, she explained.
While organocatalysts are used widely in research and discovery, they haven’t quite found their way into large scale production yet – though that doesn’t mean they don’t have the capacity. For example, the industrial synthetic route to antiviral oseltamivir has 12 steps, while an alternative organocatalytic route only has five.
What did the laureates do?
List and MacMillan started to explore using small organic molecules to bring about asymmetric reactions at the beginning of the 21st century, a time when enzymatic and metal catalysis were all chemists knew. While there were some early examples of asymmetric organocatalysis, notably the Hajos–Parrish–Eder–Sauer–Wiechert reaction discovered in the 1970s, the idea didn’t seem gain much traction – possibly because the mechanism remained mysterious at the time.
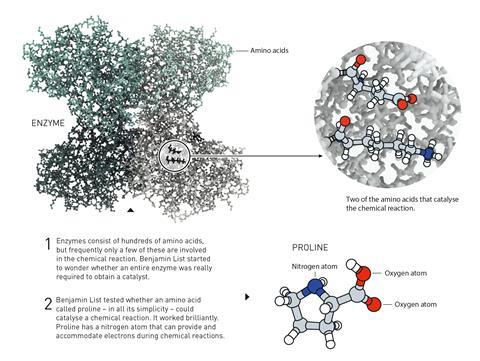
Having worked on redesigning antibodies to do chemical catalysis, List decided to try and find out what happens when biocatalysts are stripped down to their most basic chemical form. The enzyme Aldolase A performs aldol reactions – combining two carbonyl compounds to create a new carbon–carbon bond – in the body. But it is really only three amino acids that are responsible for its catalytic activity. In 2000, List and his team found out that one of them – proline – can on its own catalyse asymmetric aldol reactions with enantiomeric ratios up to 98:2.
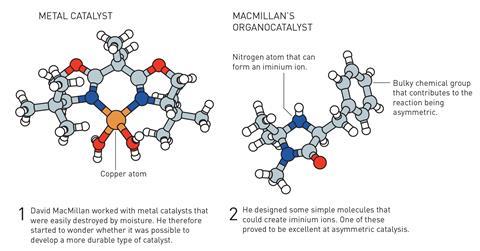
At the same time as List – though neither knew of the other’s work – MacMillan wanted to develop a catalyst for the Diels–Alder reaction, one that could overcome the drawbacks of the highly air- and moisture-sensitive metal catalysts. His team discovered that a modified phenylalanine could catalyse asymmetric reactions between α,β-unsaturated aldehydes and dienes with enantiomeric ratios up to 98:2.
When List did his initial experiments, he thought ‘maybe it’s a stupid idea, or somebody has tried it already’, he recalled in a phone call with the Nobel committee. ‘When I saw it work, I did feel like it could be something big.’
How does it work?
Organocatalysts bind to the reacting molecules to form short-lived intermediates that are more reactive than the substrate molecules on their own. Being chiral, the catalyst transfers its handedness to the substrate, controlling which side of the intermediate can react further.
In his 2000 work, List and his team described proline as a micro-aldolase, an enzyme mimic that combines a nucleophilic centre (the amino group) and an acid–base co-catalyst (the carboxylic acid group). In the aldol reaction, proline binds to the ketone, forming an enamine intermediate that is more reactive than the ketone itself, as its highest occupied molecular orbital (HOMO) is higher in energy. Proline’s handedness then infuses the reaction with asymmetry by only allowing the aldehyde to approach in one position.
In contrast, the phenylalanine derivative from MacMillan’s work reacts with the unsaturated aldehyde to form an iminium ion, whose lowest unoccupied molecular orbital energy is lower than that of the aldehyde – again making it more reactive. As in the case of List’s enamine catalysis, the catalyst attaches itself to the substrate with a reversible covalent bond, which allows it to transfer its chirality onto the reagents.
Why wasn’t it developed sooner?
Achiral organocatalysts have been around for centuries, with an early example coming from Justus Liebig, who in 1860 reported that acetaldehyde catalyses cyanogen hydrolysis. Over the course of the 20th century, there were some reports of organic molecules acting as asymmetric catalysts (with varying success). But they were isolated, curious examples. Nobody thought of developing a comprehensive methodology or understanding of how they work.
Sometimes, the most obvious answers are obscured by our preconceptions about how the world should work – and the simplest solutions can simply be too obvious, said Peter Somfai, member of the Nobel prize committee. ‘I’m an organic chemist, I’m working with small organic molecules every day but I didn’t think of it!’
What did the discoveries lead to?
The field has come a long way since its humble beginnings. The laureates’ surprising discovery that small organic molecules can catalyse complex enantioselective reactions – and the simple explanations of how they do this – led to an explosion of research in the area, said Somfai. Today, organocatalysis is recognised as the third pillar of asymmetric catalysis, next to enzymatic and transition metal catalysis. List, MacMillan and a host of chemists inspired by their work, are discovering more and more organocatalytic reactions, including organocatalysed versions of classic asymmetric reactions, such as Mannich reactions, Michael additions and Friedel–Crafts reactions. Organocatalysis has even been merged with another green corner of chemistry: photocatalysis.
‘The real revolution of our discovery is only surfacing now with extremely reactive catalysts that can do stuff that you cannot do with enzymes or even with the most sophisticated metal complexes,’ said List.



















No comments yet