Forget fluorescent light bulbs, says James Mitchell Crow. As the field of photochemistry has become dramatically more sophisticated over the past 10 years, so has the reactor technology used to drive it
For a reaction he’s spent the past decade ‘running pretty badly’, David MacMillan has achieved some pretty impressive feats with photoredox chemistry. This branch of photocatalysis was, if not born, then spectacularly reborn in his Princeton University laboratory in 2008.1 MacMillan and his team picked up photoredox catalysis and sprinted with it. Almost a decade later, they’re still pushing the science in innovative new directions – publishing eight new Science and Nature papers just since 2015.

But the publication that might ultimately prove as important as all these combined appeared in April 2017.2 In that paper, MacMillan and his long-time Merck & Co collaborator Ian Davies, assisted by a team of Merck engineers, described their design for a standardised research-scale photoreactor. Designed to direct as many of the LED’s photons into the reaction vial as possible, the device shows just how inefficient simply aiming LEDs at a flask is. Reactions in the photoreactor are completed up to 10 times faster.
The reactor design is freely available: you can download the parts list and the 3D printer file of the casing, and assemble one yourself. Or you can join the queue at one of the companies starting to build and sell them. And if that device doesn’t quite meet your needs, other reactor designs are also in the wings, from multi-well designs for visible light photocatalysis, to 5000-watt UV photochemical flow reactors that can produce kilograms of product per hour.
Things are looking bright for organic photochemistry.

Light bulb moment
Back in 2008, when MacMillan published his first photoredox reaction, sophisticated reactor designs were far from his mind. He deliberately kept the reaction setup as simple as possible. ‘At the beginning, I was convinced if we didn’t use just normal household light, no one would ever use this chemistry,’ MacMillan says.
For MacMillan and his then-postdoc David Nicewicz, the photoredox story started with a reaction powered by a standard household compact fluorescent light bulb. The team was trying to find an efficient way to asymmetrically alkylate an aldehyde at the alpha position – a highly sought transformation that nobody seemed to be able to make work.
‘Initially I was working with UV light to do that reaction,’ says Nicewicz, now an associate professor at the University of North Carolina at Chapel Hill, US. ‘It worked, but not terribly well, and Dave [MacMillan] challenged me to think about it some more and come up with something better.’
MacMillan also worried that if the reaction required high energy UV light, the need for specialised equipment would mean nobody would ever use the reaction anyway.
‘It was one of those reactions where you draw it up in your lab notebook and think, there’s no way this’ll work’
Dave Nicewicz
‘So I asked Dave Nicewicz if he could think of a way of doing this reaction that didn’t involve high energy UV light,’ says MacMillan. ‘When I said that, I didn’t mean go and find a way to use low energy light,’ he adds. But Nicewicz had done his PhD at the University of North Carolina at Chapel Hill, where there was a lot of work using photoredox catalysts for solar energy capture. Using the same visible-light-capturing ruthenium catalyst seemed worth a try.
‘It was one of those reactions where you draw it up in your lab notebook and think, there’s no way this’ll work,’ Nicewicz says. ‘And it ended up working extremely well on reaction number one, which of course never happens. I drew up the catalytic cycle on the board, and Dave immediately saw the promise in that,’ Nicewicz adds. ‘But I don’t think either one of us knew how big this was going to become.’ Or that they’d both still be focussed on it, 10 years later.
Single electron shuffle
Photocatalysis has seen an exponential growth in interest, and in publications per year, over the last decade, the vast majority of which are photoredox reactions. On one level, the reason why is pretty straightforward, says Oliver Reiser, who researches photochemistry at Regensburg University, the home of Germany’s training centre for photochemistry. ‘Photoredox simply allows you to do a lot of transformations you were not so easily able to do before.’

At the heart of the process is typically a light-sensitive iridium or rhodium organometallic species that can capture visible light photons, a process that kicks one of its electrons into an excited state. Once excited, that photoredox catalyst can start bouncing electrons around: it can donate that single electron, or the same species now has a space in a low energy orbital so alternatively it can receive an electron.
Trading in single electrons, photoredox catalysts turn out to be a very clean and controlled way to generate free radicals. ‘Radical chemistry was really popular in the 1980s and 1990s, it was a great way to do complex cascade reactions,’ says Kevin Booker-Milburn, a photochemical reactor pioneer at the University of Bristol, UK. But it never made it into industry because the reaction required tributyltin hydride as the radical initiator, a highly toxic molecule the pharma industry wouldn’t go near. Photocatalysis has brought radical chemistry back to the table, Booker-Milburn says.
‘If you wanted to just try something once or twice … you would instantly shy away. Today, anybody can buy the necessary equipment for just a few euros’
Oliver Reiser
Photoredox catalysis has turned out to be good for C-H functionalisations, for example. ‘Photoredox is not just about making radicals,’ says MacMillan ‘Its bouncing those electrons around – you’ve created a different reaction environment where you can give and take electrons, with this huge driving force, at any given moment.’
As MacMillan is the first to declare, photoredox had been around for a while when he and Nicewicz came upon it. ‘If you go back in the literature, there were two or three groups in the 1970s doing photoredox, and the field didn’t really take off, I don’t know why.’
For Reiser, one key reason is technology: the availability of cheap, powerful LEDs. ‘When I did my PhD in the late 1980s and early 1990s, [to do photochemistry] you had to have expensive equipment – immersion lamps which required cooling and was not easily done on small scale, or Rayonet reactors – it was immediately a big investment,’ Reiser says. ‘If you wanted to just try something once or twice because you had an idea for photocatalysis, you would instantly shy away. Today, anybody can buy the necessary equipment for just a few euros.
‘And then of course there were very spectacular reactions that came out at the same time,’ Reiser adds. ‘I think the really great original contributions of Dave MacMillan, and people like Corey Stephenson [at the University of Michigan, US] and others who had some early examples, really triggered it.’
Rays of reactivity

Operating in a different mode to traditional ion-based organic chemistry, and under mild conditions, photoredox chemistry allows easy, clean access to sets of structures that were hard or impossible to make before – including late stage functionalisation of already complex molecules. That’s a highly appealing proposition for the pharmaceutical industry, where new structures means potential new bioactivity and drug leads.
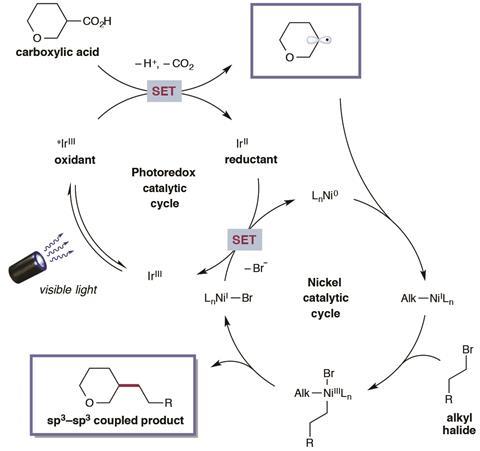
Among their current libraries of compounds, one particularly vast set are compounds incorporating carboxylic acids. In their light-excited state, photoredox catalysts have proven to be very good at plucking an electron from the carboxylic acid, generating a radical that rapidly decarboxylates.
Stop the process at that point, and you’ve generated a huge new library of compounds with their carboxylic acids snipped off, to test for bioactivity. But add a second catalyst to the reaction mixture, and you can capture the decarboxylated radical and pull it into a cross coupling reaction with an alkyl halide,3 making even more use of that vast carboxylic acid library to generate new structures.
One of the most effective ways pharma has found to make a bioactive molecule more drug-like, improving its stability in the body and its pharmacokinetics, is to incorporate fluorine, especially the trifluoromethyl group (CF3). Chemists have developed several potent methods to introduce CF3 groups at small scale, but the reagents are too expensive or cannot be made in sufficient quantity to scale the reaction up to the level pharma needs for preparative-scale work.
‘Corey Stephenson doesn’t get all the press, but he’s a hero’
Kevin Booker-Milburn
One of Stephenson’s key contributions to the field has been to use photoredox to overcome this limitation.4 The cheapest, most readily available potential source of CF3 is trifluoroacetic anhydride (TFAA), derived from trifluoroacetic acid – but it required harsh conditions to decarboxylate the compound to release CF3. Stephenson used a photoredox catalyst to decarboxylate the TFAA, generating the CF3 radical, which can attach to a range of organic substrates.
Stephenson even came up with a flow reactor design to run the reaction at kilogram scale.5 ‘Corey Stephenson doesn’t get all the press, but he’s a hero,’ says Booker-Milburn (see box).
Photoredox helpline
One measure of photoredox chemistry’s uptake by the wider community was the number of requests for help coming through to the field’s big names. ‘In terms of pharma, the discovery and medicinal chemistry side has fully adopted it as far as I can tell. I get a lot of requests to advise on that,’ says Nicewicz.
MacMillan was starting to get so many emails from folks seeking advice on how to get this reaction or that reaction working, ‘it was becoming almost like a helpline for photoredox,’ he says. ‘I was sitting with Ian Davies from Merck having a coffee and Ian said, “we have these really great engineers at Merck, why don’t we get together and try to come up with a standard system that uses standard vials”.’
‘Along the way the engineers said, you’ve actually been running these reactions pretty badly, because you’re only getting a small percentage of the photons from these lamps,’ MacMillan adds. In the final design, the vials hang 6mm above the LED array, within a cylindrical mirrored housing that stops photons from escaping, reflecting any photons that bounce off the vial back into the mix.
Independently, Nicewicz has a 16-well reactor of similar scale in the wings. ‘Scaling up and being able to do large scale reactions is the next problem, which a lot of people are working on currently,’ he says. ‘I think it’s just a matter of time before a combination of chemists and chemical engineers will solve that problem. And pretty easily, honestly, I don’t think it’s a major limitation.’
Switching metals
But the focus on reactor design isn’t slowing the dazzling array of new chemistry continuing to come in photoredox chemistry and neighbouring areas.
‘It’s something different … I think this will become an active area pretty quickly.’
Daniel Weix
From the beginning, iridium and ruthenium complexes have been the light-gathering organometallics of choice, but now the spotlight is beginning to fall on other metals. Reiser has a particular focus one copper photocatalysts. ‘Initially we thought copper might just be an economic alternative to ruthenium and iridium. But more recently we have really strong evidence copper works in a unique way,’ Reiser says.6 ‘Beyond electron transfer, the copper is also getting involved as a transition metal into the catalytic cycle. We see that more and more.’
Switching focus entirely, Todd Krauss and Daniel Weix at the University of Rochester, US, recently showed light-harvesting quantum dots can catalyse photoredox reactions.7 ‘It’s something different – so they might offer opportunities you just can’t do with a metal complex,’ says Weix. ‘I think this will become an active area pretty quickly.’
A decade after he began dabbling with photoredox, MacMillan isn’t running short of new ideas. Pairing the photoredox catalyst with another organometallic catalyst is opening up new areas of reactivity, he says.
For example, he’s using dual metal reaction systems in which the light-harvesting complex funnels the captured energy into a transition metal catalyst that then engages with the substrate.
‘Imagine there’s some transition metals that could do oxidative addition, or could do reductive elimination – but you’d have to heat them up to, say 2000°C,’ MacMillan says. If you did that, everything in the flask would disintegrate – not very useful.
‘What’s happening with photochemistry is, you’ve got about 60kcal of energy mainlined into the transition metal catalyst without touching anything around it. We’re finding you can dramatically expand the range of reactions you can do by having a photocatalyst that can transfer its energy into the organometallic transition metal catalyst to do lots of things it wouldn’t do before,’ MacMillan says. The team has already published some examples with nickel and is already looking at other metals.8
‘In retrospect it’s obvious you should be able to do this but I don’t think many people had thought about it, we certainly hadn’t,’ MacMillan says. ‘Once you see what you can do, you realise the world’s your oyster.’
All the light we cannot see
Kevin Booker-Milburn from the University of Bristol only did one UV-powered photochemical reaction during his postdoc in the late 1980s. But it made an impression.
Whereas most organic molecules are transparent to visible light, many absorb UV wavelengths – especially molecules incorporating double or triple bonds. Under UV irradiation, these molecules can often be coaxed to join together via cycloaddition reactions. ‘What stuck with me was the fact it’s completely reagent-less – no catalysts even, just UV light – but you made very complex molecules in one step just by fusing two things together, in a way you couldn’t even do catalytically,’ says Booker-Milburn.
‘When I became an independent lecturer in the early 1990s, that was one of the areas I started to play with as a tool for natural product synthesis.’ He’s been doing so ever since – and trying to persuade pharmaceutical industry chemists to do likewise. ‘I was always trying to convince pharma these are wonderful molecules they should investigate as leads in drug discovery,’ Booker-Milburn says. But he always got the same reply: it’s difficult to do and you can’t scale it up.
Photochemistry’s Achilles heel was the limited distance light penetrates into a solution – an insignificant problem with milligram-scale reactions done in little flasks, but a show-stopper in bigger flasks. So teaming up with Malcolm Berry at GlaxoSmithKline, Booker-Milburn started to look at flow chemistry, building a custom-designed photochemistry flow reactor.

The key part of the design was fluorinated ethylene propylene (FEP) tubing wound around a UV lamp. The solution of reactants was bathed in photons as it repeatedly circumnavigated the lamp through the tubing. ‘In 2005 we published the first practical flow reactor for photochemistry,’ Booker-Milburn says. ‘We could get hundreds of grams of product in 24 hours.’9
The reactor got a shot of publicity when Peter Seeberger from the Max-Planck Institute for Colloids and Interfaces, Germany, used essentially the same design in his pioneering flow synthesis of the malaria drug artemisinin.10 ‘From then people started to pick up on it,’ Booker-Milburn says.
Last year , Berry, Booker-Milburn and their collaborators published a bigger, better design, which replaced the degradation-prone FEP tubing with quartz tubes.11 The tubes surround a UV lamp that goes up to sizzling 5000 watts. Needless to say, the reactor incorporates water- and air-cooling, encased in a reflective metal sleeve to keep the powerful photons caged.
‘It’s the size of a rotary evaporator condenser, yet it can churn out kilograms per day,’ Booker-Milburn says. ‘So we really are at the process size now and there’s been a lot of interest.’
It’s possible to swap out the UV lamp for a visible light source to do photoredox reactions at scale – but a custom-designed reactor would be a better solution, he adds. ‘I’ve joked to Dave MacMillan that photocatalysis is taking photochemistry and making it more difficult!’ Booker-Milburn says. ‘Because you’re throwing in a catalyst, which by its nature has to absorb a lot of light – they’re such phenomenal light gatherers, the light doesn’t really penetrate very far into the solution,’ he says. ‘If you want to process litres of stuff you have to have good light penetration.’ The reactor design he’s working on now has ‘maybe a few tricks to get round that problem’.
References
1 D A Nicewicz and D W C MacMillan, Science, 2008, 322, 77 (DOI: 10.1126/science.1161976)
2 C Le et al, ACS Cent. Sci., 2017, 3, 647 (DOI: 10.1021/acscentsci.7b00159)
3 C Johnston et al, Nature, 2016, 536, 322 (DOI: 10.1038/nature19056)
4 J W Beatty et al, Nat Commun., 2015, 6, 7919 (DOI: 10.1038/ncomms8919)
5 J W Beatty et al, Chem, 2016, 1, 456 (DOI: 10.1016/j.chempr.2016.08.002)
6 D B Bagal et al, Angew. Chem., Int. Ed., 2015, 54, 6999 (DOI: 10.1002/anie.201501880)
7 J A Caputo et al, J. Am. Chem. Soc., 2017, 139, 4250 (DOI: 10.1021/jacs.6b13379)
8 E R Welin et al, Science, 2017, 355, 6323, 380 (DOI: 10.1126/science.aal2490)
9 B D A Hook et al, J. Org. Chem., 2005, 70, 7558 (DOI: 10.1021/jo050705p)
10 F Lévesque and P H Seeberger, Angew. Chem., Int. Ed., 2012, 51, 1706 (DOI: 10.1002/anie.201107446)
11 L Elliott et al, Org. Process Res. Dev., 2016, 20, 1806 (DOI: 10.1021/acs.oprd.6b00277)









No comments yet