Structural innovations are overcoming oligonucleotide drugs’ historical flaws, discovers Andy Extance

Few patients would want a drug injected directly into their eyeball, but even that can be preferable when the alternative is losing their sight. Similarly, few drug companies would want to deliver products in such a traumatising manner, but when they’re pioneering a new technology, better treatments will follow – or so they might hope.
Isis Pharmaceuticals of Carlsbad, California, has been working on improving matters since before Vitravene (fomivirsen), the first antisense oligonucleotide drug, even gained approval in 1998. Developed to treat a potentially blinding viral condition afflicting HIV sufferers, it provided both industry and academia with a real example of the potential these DNA-like chains embody.
The technology’s early incarnations brought struggles that resulted in Vitravene’s unusual administration route. Oligonucleotides’ chemical structures hamper their access to where they do their biological job and can also trigger strong, and undesirable, immune responses.
For 15 long years it looked like researchers might lose the battle. Vitravene was discontinued in 2004, and all of its successor antisense drugs failed to reach the market – until January 2013. That was when the US Food and Drug Administration (FDA) approved Isis’s drug Kynamro (mipomersen) to treat homozygous familial hypercholesterolemia, a rare genetic condition leading to high cholesterol levels.
Today, several companies have further oligonucleotide drugs nearing approval decisions for genetic-origin ailments. Targeting such diseases with oligonucleotides reduces the risks of drug development failing, claims Frank Bennett, senior vice-president of research at Isis. He hopes an understanding of historical challenges has advanced enough for that benefit to now shine through. ‘Our failure rate historically was comparable to industry standards,’ Bennett asserts. ‘Our hope is that’s now improved.’
All go for oligos?
The very name ‘oligonucleotide’ is a clue to where their promise originates. ‘Oligo’ means a few, and the nucleotides in question are the DNA and RNA bases, the fundamental building blocks of an organism’s genetic code. A single oligonucleotide strand containing around 20 bases in the right sequence can pair up with any gene, a fact that is already widely exploited by microarray chips full of such molecules used in DNA analysis.
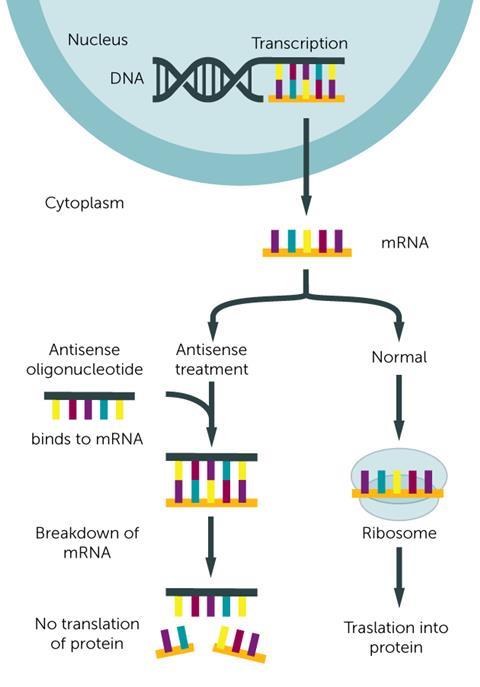
Molecular biologists call a single DNA coding sequence carrying the inherited genetic instructions for biological synthesis of a protein a ‘sense’ strand, and its partner sequence is ‘antisense’. Rather than DNA, antisense oligonucleotide therapies target the RNA derived from it, which carries the messages controlling protein synthesis. Vitravene, for example, binds to cytomegalovirus messenger RNA (mRNA), stopping it making essential proteins.
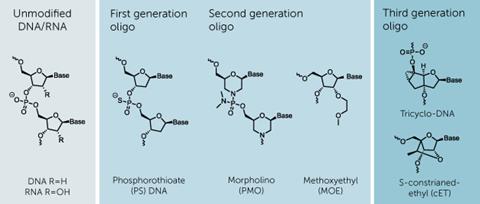
The major difference between oligonucleotides and natural nucleic acid chains is the structure between the bases. To be stable against nuclease enzymes that break down DNA and RNA, oligonucleotides must use something other than the natural phosphodiester-linked sugar units.
Vitravene had phosphorothioate linkages, where one of the oxygen atoms in the phosphodiester is replaced with a sulfur atom. Sulfur is more hydrophobic, making these oligonucleotides more likely to attach to molecules other than RNA. When phosphorothioates are administered in the blood, it causes more than 85% of their molecules to bind to proteins like albumin.1 Such protein interactions can help, stopping the drugs being urinated out too quickly, but also risk unwanted side-effects. Sulfur’s presence can also produce toxic interactions, depending on the oligonucelotide sequence, potentially slowing clotting, while promoting inflammation and immune responses.2 Being injected straight into the eye, and avoiding the bloodstream, meant Vitravene sidestepped any problems.
One oligonucleotide currently awaiting an FDA decision is drisapersen, a phosphorothioate with a methyl group added to the 2’-hydroxyl group of ribose, sponsored by BioMarin Pharmaceutical. The company, based in San Rafael, California, hopes to get a decision on the use of drisapersen to treat Duchenne muscular dystrophy (DMD) by the end of the year, says vice-president Scott Clarke. DMD is a deadly disorder caused by mutations that prematurely stop production of the protein dystrophin, which forms connections to muscle fibres. Children with DMD, almost exclusively boys, are typically unable to walk by the time they turn 12 years old and die from suffocation in early adulthood as their heart and lung muscles give out.
Rather than binding mRNA as antisense oligonucleotides traditionally do, drisapersen sticks onto longer pre-mRNA chains, sterically blocking the mutation and its surrounding sequence. That induces ‘exon-skipping’ that prevents the section, or exon, containing the mutation being spliced into the mRNA that feeds sufferers’ protein synthesis machinery. While the shortened dystrophin that results isn’t perfect, it’s far better than the alternative.
To ensure dystrophin production throughout patients’ bodies, drisapersen will be injected under their skin at various sites in rotation. Trials have shown protein is made, though there isn’t agreement about how much or where it’s required. ‘We’re focussed on demonstrating the drug has a clinical benefit, so the exact location of dystrophin expression becomes less important,’ Clarke says.
Drisapersen’s modified structure offers improved nuclease resistance and reduced toxicity compared to first generation phosphorothioates, and consequently Biomarin has found that it is ‘generally well tolerated’, according to Clarke. The most significant side-effects include reactions like redness and swelling at injection sites and protein in patients’ urine. The latter is a possible kidney damage indicator, but is seen in a minority of cases and hasn’t caused serious medical issues, Clarke explains.
Replacement backbones
Building on the original exon-skipping technology, Sarepta Therapeutics is using strands that substitute DNA’s deoxyribose sugars for morpholine rings, and DNA’s phosphodiesters for phosphorodiamidates. The resulting phosphorodiamidate morpholino oligomers (PMOs) are very resistant to degradation, meaning they can be given in high doses with few side-effects. However, the more complex structure is harder to make and therefore likely to be more expensive, depending on production scale. Sarepta, based in Cambridge, Massachusetts, is awaiting an FDA approval decision for its DMD drug eteplirsen, which it hopes will be made by the end of November.
We see a 10-fold improvement in potency
Because Sarepta and Biomarin both use exon-skipping and address the same disease, they’re battling over who owns the patent rights. With approvals potentially imminent and the urgent need in DMD, Sarepta’s interim chief executive Edward Kaye is keen to provide patients with a treatment as soon as possible. ‘No matter the legal decision, which is expected in the next few months, it’s likely there’ll be an appeal and that will take an additional 12–18 months,’ he says. Kaye adds that negotiations between the companies might shorten that process.
Rapidly learning from its experiences with Vitravene, Isis has also adopted ‘second generation’ backbone chemistries to make it easier to administer drugs via the bloodstream. Its cholesterol-reducing drug Kynamro and most of its current drug pipeline feature sugars modified with extra 2’-O-methoxyethyl substituents at both ends and 10 deoxyribose sugars in the middle.
‘We see about a 10-fold improvement in potency over first generation chemistry, and an improvement in stability in biological tissues such that it has a longer duration effect,’ Bennett explains. ‘That allowed us to go from daily to weekly injections. It also has a much better tolerability profile when administered systemically.’
The middle deoxyribose sugar section means these molecules are referred to as ‘gapmers’. Enzymes like Ribonuclease H that normally cut up RNA when still bound to DNA in natural replication processes can do the same when mRNA is bound to the gapmers. ‘Ribonuclease H recognises the DNA/RNA heteroduplex and causes the degradation of the RNA, releasing the DNA, or in our case the oligonucleotide, to bind to other RNA,’ Bennett says. ‘It’s a catalytic mechanism.’ In Kynamro, this destroys mRNA in the liver that would produce a protein called apolipoprotein B that carries fat around the body.
Beyond rare diseases
Amid several Isis mRNA-degrading oligonucleotides in advanced development stages, one candidate notably doesn’t use this approach. Since August 2014, ISIS-SMNrx has entered a series of pivotal Phase III clinical trials for spinal muscular atrophy (SMA). Although it only affects about one in 10,000 people, it is the most common genetic killer of infants. In SMA, infants don’t make enough of the ‘survival of motor neuron’ (SMN) protein, meaning motor neurons progressively die off. The disease is normally caused by mutations in the gene SMN1.
However, most people have a second gene called SMN2 that mostly produces a less stable SMN protein. That is because the SMN2 gene has a very slightly different sequence, explains Adrian Krainer from Cold Spring Harbor Laboratory in Long Island, New York. Splicing repressor proteins naturally block an exon in the derived mRNA from being included in the SMN protein, making it unstable. Working with Krainer, Isis has developed an oligonucleotide drug that modulates splicing to make stable SMN2 -derived SMN protein. ‘What we do could be called exon inclusion,’ Krainer says. ‘Blocking splicing repressor proteins from binding the SMN2 RNA restores the exon.’
Delivering these drugs may heavily depend on chemistry
Kaye believes that Sarepta’s PMOs could also be used to fight SMA, but the company’s testing is at an early stage. He emphasises the broad possible range of diseases that may be targeted using oligonucleotides to manipulate RNA, likening it to a temporary, potentially safer form of gene therapy. ‘If anything goes wrong we just stop giving the drug,’ he says.
Sarepta has also developed experimental oligonucleotide therapies for the haemorrhagic fever viruses Ebola and Marburg that would function similarly to Vitravene.3 But with government funding for such studies now in decline, its focus has shifted to antibiotic resistant bacteria.4 ‘We target gram negative infections, making previously resistant bacteria vulnerable by knocking out genes,’ Kaye explains.
Kaye highlights cancer as another potential application. Isis is already active in this area, with custirsen, an oligonucleotide it helped develop to block the synthesis of a protein key to cancer’s survival, in phase III trials. Among Isis’ other cancer projects, it is also working with Krainer on a new target in very early preclinical studies. ‘We’re interested in pyruvate kinase, which seems to be involved in the Warburg effect, the unique glycolytic metabolism of cancer cells,’ Krainer says. ‘The idea is to force cancer cells to make a different pyruvate kinase so that they cannot sustain metabolism.’
However, Bennett emphasises that the risks inherent in such efforts are greater than for genetic diseases. This is exemplified by the phosphorothioate oligonucleotide oblimersen, which targets mRNA encoding a protein that many cancers use to hold off programmed cell death. From 1999, this drug went through at least 45 clinical trials, but ultimately the research programme was terminated and its developer Genta declared bankrupt.
‘There are unique difficulties in delivering oligonucleotide-based drugs into tumours and cancer cells,’ explains Krainer. ‘Their cell surfaces may look different, tumours may be encapsulated. So solutions need to be found to deliver these drugs, and that may heavily depend on chemistry.’
On target
‘Delivery remains the ultimate challenge for oligonucleotide therapeutics,’ says Jonathan Watts from the RNA Therapeutics Institute at the University of Massachusetts Medical School in Worcester. One aspect of this is delivery within cells. Watts underlines evidence suggesting that antisense oligonucleotides are best suited to silencing RNA in cell nuclei and that modifications to get them there better have an advantage. He highlights third-generation tricyclo-DNA backbone chemistry, which tacks two extra small rings onto DNA’s deoxyribose sugars.5 Such structures ‘preferentially localise to the nucleus’, he says.
Before they even reach the cell, oligonucleotide drugs must also reach the right body part. ‘The field has a number of technologies in early-stage clinical trials that show real potential for delivering oligos to the liver, but delivery to other tissues is still very challenging,’ Watts says. ‘Perhaps surprisingly, the central nervous system is showing a lot of promise. By infusing an oligo into cerebrospinal fluid, it can diffuse through the brain and be taken up. This is exciting in terms of neurodegenerative diseases.’
At Isis, medicinal chemistry efforts include the company’s own ‘constrained ethyl’ chemistry, looping round the 2’-substituent of its oligonucleotide sugar segments to make a bridged ring.6 ‘That further increases potency roughly around 10-fold compared to the 2’-methoxyethyl chemistry,’ Bennett says. There’s been great activity in making such modifications, for example with Sarepta’s Ebola and Marburg work exploiting its PMO-plus chemistry, replacing a neutrally-charged dimethylamine residue with a positively-charged piperazine. Sarepta and Isis are also coupling their oligonucleotides with other molecules that help them into the right cell type. One example is a carbohydrate that targets the asialoglycoprotein receptor, which Bennett says gives Isis an eightfold improvement in potency for liver targets.
Inevitably, each new innovation adds cost, which is another long-standing perceived weakness of oligonucleotides. A 2002 report put oligonucleotide prices at $2000 per gram [£1300 per gram], but Bennett says that since Isis launched Vitravene, they’ve fallen by ‘several orders of magnitude’. The increased use of oligonucleotides in diagnostics and conventional chemical scaleup helps offset the added expense of more complex structures and targeting ligands. ‘There’s a lot of internal chemistry going on to make the process more efficient, and then we’re transferring those to CROs that are implementing them when they scale up,’ Bennett says. ‘It’s really scale that’s driving costs down.’ Higher potencies that lower the amount of drug needed also help lower prescription prices.
Watts welcomes the progress being made, but remains cautious on the technology’s short-term chances of success. ‘Oligonucleotide therapeutics have widely been seen as being “about to deliver” for some 20 years,’ he warns. ‘I’m hesitant to promise a clinical breakthrough in the immediate term. However, it’s clear that the chemistry and delivery approaches in clinical trials today are much more sophisticated and effective than even two to three years ago.’
But oligonucleotide developers remain convinced the technology will eventually become a productive platform for a wide variety of new drugs. They give the example of monoclonal antibodies, which took decades to develop, but are now taking the pharmaceutical industry by storm. ‘Small molecule drugs have 125–150 years of history, antibodies have had close to 50 years of evolution,’ says Bennett. ‘We’re about 25 years in. By no means is the technology plateaued out. There will be further innovations and developments that enhance its therapeutic potential and convenience.’
Andy Extance is a science writer based in Exeter, UK
References
1 R S Geary et al, Adv. Drug Deliv. Rev., 2015, 87, 46 (DOI: 10.1016/j.addr.2015.01.008)
2 A M Krieg et al, Nature, 1994, 374, 546 (DOI: 10.1038/374546a0)
3 P L Iversen et al, Viruses, 2012, 4, 2806 (DOI: 10.3390/v4112806)
4 B L Geller et al, J. Infect. Dis., 2013, 208, 1553 (DOI: 10.1093/infdis/jit460)
5 D Ittig et al, Nucl. Acids Res., 2004, 32, 346 (DOI: 10.1093/nar/gkh187)
6 P P Seth et al, Nucl. Acids Symp. Ser., 2008, 52, 553 (DOI: 10.1093/nass/nrn280)











No comments yet