Using dental calculus from Neanderthals and Palaeolithic humans, researchers have reconstructed ancient microbial genes and engineered modern bacteria to produce their previously unknown metabolites. The approach will allow natural product researchers to ‘add a new dimension and go back in time’ according to bioorganic chemist Pierre Stallforth from the Hans Knöll Institute in Jena, Germany, who led the project.
Microbial metabolites play essential biological roles, but their chemical breakdown over time makes it difficult to study those from ancient sources. Now, Stallforth’s team has used palaeogenomic techniques to reconstruct ancient microbial genomes and resurrect their biosynthetic pathways. With this approach, researchers can access previously hidden structural and functional diversity and learn about the environments the microbes inhabited.

The team recovered DNA from palaeological samples of bacteria that lived inside the mouths of Neanderthals and ancient humans. Using these DNA fragments, the researchers could reconstruct metagenomes of the ancient bacteria. Stallforth likens the process to assembling a book from torn-up pieces of each page.
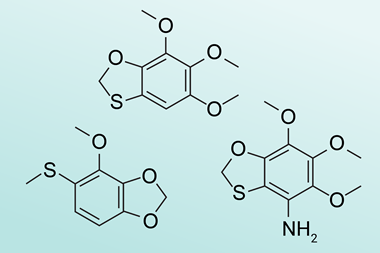
The bacteria included several species of Chlorobium that shared one particular biosynthetic gene cluster that was responsible for the production a previously unknown class of metabolite. The team then engineered modern bacteria to reproduce the Chlorobium’s chemical products, which they named paleofurans. This work ‘shows it’s possible to take ancient genetic material and get functional information out of it that allows you to produce a compound’, says Stallforth.
‘[The work] demonstrates a completely new way of how we can think about biological diversity or functional diversity – not just from the perspective of what is today … but from the perspective of what has been in the past,’ comments Katerina Guschanski, a genomics researcher from the University of Edinburgh, UK , who was not involved in the project. ‘As with every technological innovation, it will shift the landscape quite considerably of what you can do.’
Gaining access to the chemical diversity of the past could aid the discovery of new functional molecules. For example, Guschanski suggests that the approach could help uncover new antibiotics that might aid in the fight against antimicrobial resistance.
Stallforth notes that the project also raises ethical questions regarding ancient DNA, adding that he hopes the work spurs discussions on ‘what a good approach is to deal with intellectual property that stems from ancient human beings or Neanderthals’.
References
M Klapper et al, Science, 2023, DOI: 10.1126/science.adf5300














No comments yet