Researchers have demonstrated for the first time that fluorine atoms can quantum tunnel. As 100 years of quantum mechanics is celebrated this year, this breakthrough opens new avenues for controlling chemical reactions and better understanding of the chemistry of fluorinated compounds.

Quantum tunnelling allows a particle to effectively pass through an energetic barrier that it doesn’t have enough energy to surmount. It’s the result of the probabilistic nature of quantum mechanics that is most noticeable for very small particles with little mass such as electrons or hydrogen atoms. For these particles, there is a small chance that they could be found on the other side of, or even within, a barrier – and they routinely are. Despite the oddness of this effect it is an everyday occurrence and is critical to real-world applications such as nuclear fusion and scanning tunnelling microscopy.
So far, the largest atoms known to tunnel are oxygen and nitrogen, but scientists from the Free University of Berlin, together with colleagues in France, have now shown the first experimental and theoretical evidence for quantum tunnelling for fluorine. Their finding solves a problem that had stymied them for 15 years.
‘We were aiming to investigate transition metals in higher oxidation states,’ says Sebastian Riedel, a corresponding author on the paper. Riedel’s team were using laser ablation experiments to generate metal fluorides and trapping the products in cryogenic matrices such as solid neon to study them with IR spectroscopy. ‘And we noticed we always had one signal in our IR spectra which did not belong to a metal … it was clear it cannot be a metal fluoride … a species has been formed out of neat fluorine atoms,’ says Riedel. ’ After further experiments and simulations, they identified that the were associated with a non-metal interaction and hypothesised that it could be an exotic polyfluoride ion, F5-.
Now, the team has shown that the splitting of the IR peaks they observed is actually caused by the central fluorine atom in the F5- tunnelling, spontaneously transforming the system between two states of the polyfluoride ion. ‘We were now able to simulate the whole anion, F5-, in the matrix cavity of neon atoms. And we found the agreement between experiment and theory,’ explain Riedel.
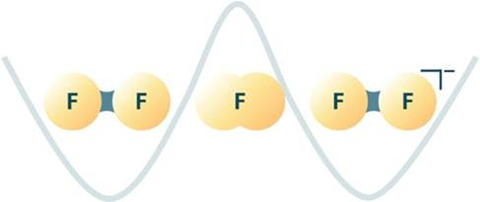
‘I predicted theoretically that some [fluorines] will tunnel, but this is huge. It is the heaviest atom that has been seen tunnelling in an experimental chemistry situation,’ says Sebastian Kozuch, a heavy atom tunnelling researcher at Ben-Gurion University, Israel. He applauds the work, impressed by the effort in finally interpreting inexplicable spectra from their early work.
Tunnelling probability is dependent on the mass of the particle and the size of the energy barrier. So although fluorine is large, the barrier is small because ‘in a polyfluoride anion you have a lot of electrons in a very small volume … the bonds are very weak’, explains Riedel, and this explains why they are actually separated by an energy barrier in these very specific experimental conditions. The neon matrix surrounding the molecule also plays an important role because the neon atoms ‘cage’ the fluoride ion and this puts ‘some pressure on the molecule which induces the tunnelling process by lowering the energy barrier’, explains Riedel.
Riedel says that this work is of more than just fundamental chemical interest. Fluorinated compounds are commonplace in drugs, batteries and other areas of modern life yet pose a pollution risk due to their highly stable C–F bonds. Greater understanding of fluorine’s bonding is essential to deal with the problems it creates. ‘We have to understand how fluorine bonds can be activated,’ he says.
Heavier systems where tunnelling occurs are unlikely, but there are new opportunities for the researchers. ‘We have some ideas for other molecules which, perhaps, also undergo tunnelling,’ says Riedel. He adds that tunnelling processes are often underestimated in chemical reactions but is hoping that this is going to change.
The work also advances concepts around the nature of the chemical bond. ‘The matter here is to push boundaries … so in that sense, [the research] is revolutionary,’ adds Kozuch.
References
C Müller et al, Nat. Commun., 2025, 16, 4027 (DOI: 10.1038/s41467-025-59008-6)













1 Reader's comment