A common gut bacterium can make amphetamines more addictive by producing a chemical that boosts dopamine activity in the brain. By targeting this microbial pathway, it may be possible to dampen amphetamine’s effects, helping people overcome addiction.
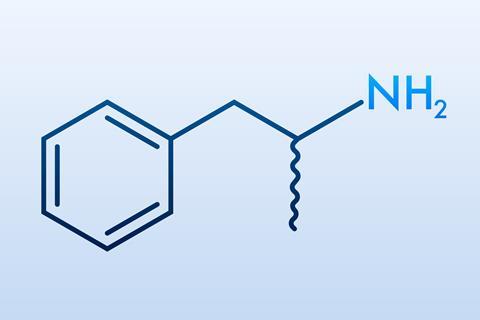
Amphetamines work by increasing dopamine, the brain’s key reward chemical. They are prescribed for certain neuropsychiatric disorders, such as attention deficit hyperactivity disorder (ADHD), but they are also widely abused. No approved drugs currently exist to treat amphetamine addiction, which is rising rapidly worldwide. Now, a team led by Angela Carter and Aurelio Galli at the University of Alabama at Birmingham, US, has shown how opportunistic gut microbes, like Fusobacterium nucleatum, can enhance the effects of these drugs, by hijacking dopamine signalling pathways in the brain.
‘In the past, people found that treating mice with antibiotics made them no longer responsive to amphetamine, but the mechanism was not clear,’ explains Galli. ‘We started to think about what these bacteria were releasing and how this might be affecting brain function.’
The gut microbiomes of people dependent on amphetamines often have higher levels of F. nucleatum, a microbe better known for causing gum disease, but can sometimes be found in the intestines. ‘There have been a lot of correlative studies showing that if you alter the microbiome, you affect a whole range of diseases, including substance use disorders,’ says Carter. ‘Researchers are just now starting to unravel the mechanisms behind this.’
When the researchers introduced F. nucleatum to the intestines of fruit flies and gave them amphetamines, they became more hyperactive than the flies given the drug alone, displaying heightened sexual motivation and a stronger preference for drug-containing food. Amphetamines on their own are known to increase activity in flies, but the presence of the bacterium amplified these behaviours. This led the team to hypothesise that the microbe was influencing the reward pathway by secreting short-chain fatty acids, particularly butyrate, which is known to cross the blood-brain barrier and affect neuronal processes.
Butyrate inhibits enzymes called histone deacetylases (HDACs), that normally help wind DNA tightly around proteins. By blocking HDACs, butyrate makes it easier to switch on certain genes. In the flies, butyrate treatment alone mimicked the bacterium’s effects, increasing expression of the dopamine transporter protein. This transporter normally reabsorbs dopamine back into nerve cells, but under amphetamine stimulation it runs in reverse, flooding the space between neurons with dopamine. More transporters mean more dopamine outflow, which is translated into stronger behavioural responses to the drug.
To confirm that dopamine levels were actually changing, the team used amperometry to measure neurotransmitter release in real time by detecting electric currents generated when dopamine molecules are oxidised at a microelectrode. This allowed the researchers to directly observe that both butyrate and F. nucleatum exposure increased the amount of dopamine released in response to amphetamines.
To further confirm the mechanism, the researchers switched off HDAC1 in dopaminergic neurons. This enhanced amphetamine-induced behaviours to equivalent levels as with either butyrate or F. nucleatum. ‘I think a lot of science is putting the pieces of the puzzle together. Little bits of information were out there but they hadn’t been linked yet,’ Carter says. ‘We tried to meticulously walk through, connecting the dots.’
When the researchers tried other gut microbes or other fatty acids such as acetate and propionate, they found no effect, suggesting that the butyrate connection is specific to F. nucleatum. They also demonstrated that the mechanism extends beyond flies, with similar changes in dopamine transporter expression observed in nematode worms.
‘In a field where many studies stop at correlations or broad antibiotic manipulations, this work contributes a tractable and mechanistic pathway by which gut bacteria can influence dopamine pathways,’ comments Erin Calipari, a pharmacologist at Vanderbilt University, who was not involved in the study.
The findings could have therapeutic implications. Antibiotics that reduce F. nucleatum have already been shown to blunt amphetamine’s rewarding effects in both animals and humans. One possibility, the team noted, is to target the bacterium with a specific bacteria-killing virus. ‘The more we understand about the mechanism, the more we can make intelligent use of it,’ explains Carter. ‘This allows us to be more logical and intentional with our therapeutic design.’
References
S J Mabry et al, Sci. Signal., 2025, DOI: 10.1126/scisignal.adx7729












No comments yet