Artificial motors’ gentle tug can activate immune cells
For the first time, an artificial molecular motor has been created that can ‘talk’ to living cells – by gently pulling their surface with enough physical force to elicit a biochemical response. The approach could help scientists decode the language that cells use to communicate with each other in tissues.
‘There is a mechanical language in the form of physical forces applied by the cells themselves, and we want to understand what information is communicated and what the consequences are,’ explains Aránzazu del Campo, who led the study at the Leibniz Institute for New Materials, Germany. ‘Ultimately, we want to be able to provide signals to cells and guide their function when they are not able to do that by themselves in disease cases.’
Usually, studying how cells communicate by sensing mechanical stimuli and producing biochemical responses requires prodding them with pipettes or the tip of an atomic force microscope. However, this doesn’t work at the more complex tissue level.
Now, del Campo’s team has shown how this could be achieved with alkene-based molecular motors that use energy from ultraviolet (UV) light to delicately pull on cell membranes with tiny amounts of force and trigger a biochemical response.
The motors comprise a stator and a rotor part, out of which protrude four polyethylene glycol arms. Motors are attached, using two of these arms, to membrane receptors of cells in culture. Meanwhile, the other two arms are tethered to a hydrogel designed to mimic a cell surface or a protein that a cell would usually interact with.
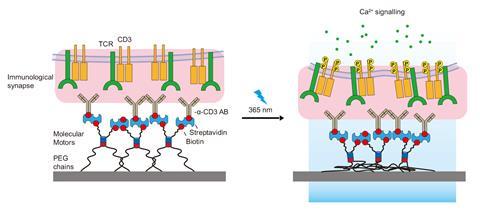
Under UV light pulses, the motors rotate, causing the two pairs of arms to coil up and shorten. They then pull on the cell membrane and trigger natural cell responses, for example enlarging their focal adhesions – macromolecular assemblies that provide mechanical links to transmit signals between cells. In another experiment, the researchers were able to use the motors to activate T cells, which are essential for the immune system. ‘We could rescue functions that cells have lost, or trigger processes that cells are no longer able to undergo,’ says del Campo.
‘This strategy to integrate motors in living systems has lots of potential,’ comments Nathalie Katsonis at the University of Groningen in the Netherlands. ‘It is an elegant approach that shows some similarities with biological strategies – in the sense that it involves a mechanical step that connects the light cue with the complex function.’
Del Campo hopes the approach will lead to better understanding of cellular function, not just in cell cultures but in living systems and whole tissues. ‘Our next goal is to couple the motor to a force sensor to quantify the force ranges at which cells talk to each other,’ she says.
References
Y Zheng et al, Nat. Commun., 2021, 12, 3580 (DOI: 10.1038/s41467-021-23815-4)










No comments yet