Researchers have created a subset of rhodamine-based structures bearing 3-phosphonates that plugs a gaping hole in the literature on these important fluorophores.1 The new compounds are also much more water soluble than conventional 3-substituted rhodamines used for cellular imaging.
Since their discovery in 1887, rhodamines’ applications have evolved from paint manufacturing and fabric dying to chemosensors and fluorescent probes for observing biological systems. And chemists have been modifying their structure to create new analogues with better properties for well over 100 years.
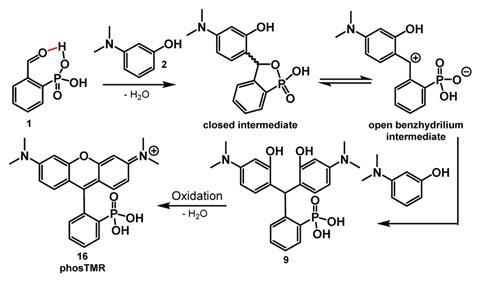
3-carboxy- and 3-sulfono-rhodamine derivatives already exist. Now, Evan Miller and colleagues at the University of California, Berkeley in the US have devised a mild and scalable method for making 3-phosphonorhodamines. ‘No one had made these compounds before. And we were surprised because it seems like an obvious modification to try. One of the joys of science is making – or describing or uncovering – something that no one else has done before,’ comments Miller.
Friedel–Crafts condensation with an exogenous acid is the traditional synthetic route for producing rhodamine analogues. The acid activates an aldehyde to promote a nucleophilic attack. Miller’s team, however, took an alternative route. They adapted a method first used to synthesise 3-carboxyrhodamines.2 It omits the need for an exogenous acid and instead uses 2-phophonobenzaldehyde, which undergoes intramolecular aldehyde activation. Reacting 2-phophonobenzaldehyde with a series of anilines forms triarylmethanes that subsequently dehydrate and oxidise to yield 3-phosphonorhodamines.
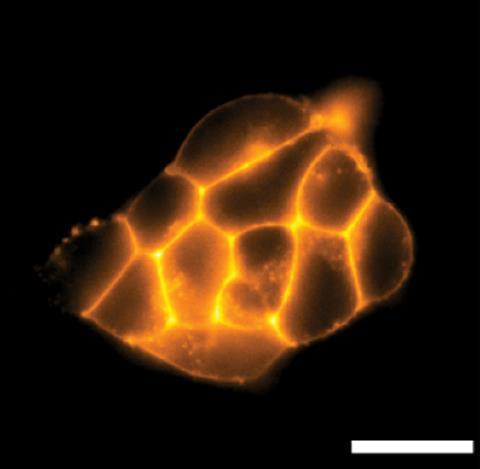
The team found that tetramethyl-3-phosphonorhodamines (phosTMR) in particular have excellent chemical stability. What’s more, they describe phosTMR as being 12 and 500 times more soluble than their 3-carboxy and 3-sulfono counterparts, respectively. Fluorescent probes need to be water soluble to access biological systems, but enhanced water solubility also benefits the fluorophores’ brightness. Here, brightness is a measure of the number of photons emitted upon light absorption. During cellular imaging, a fluorophore’s brightness decreases as the number of unreacted reagents increases. PhosTMR’s excellent water solubility makes it easier to wash away those molecules that don’t react, improving the signal of those that do. ‘Our hope is that by washing away the unreacted dye, the experiment becomes more like turning on a flashlight at night-time – the protein of interest is very noticeable. If you can’t wash away excess dye, the experiment is more like turning on a flashlight during the day – hard to pick out from the background,’ explains Miller.
Stronger signals mean you can use less dye, which in turn means protein labelling, live cell imaging, and next-generation sequencing using such dyes becomes more cost-effective. ‘There is a deficiency in water soluble emissive dyes for cellular imaging and the incorporation of the phosphate group, to increase the water solubility in the dyes, is beneficial to the field,’ comments Colleen Scott, an organic chemist from Mississippi State University in the US.
Although the reason behind the enhanced water solubility is still unknown, Evan’s team suggest it might be because phosphonic acid has two ionisable sites, whereas carboxylic and sulfonic acid only have one.
References
1 J Turnbull, et al, Chem. Sci., 2023, DOI: 10.1039/d3sc02590j
2 S J Dwight and S Levin, Org. Lett., 2016, 18, 5316 (DOI: 10.1021/acs.orglett.6b02635)









No comments yet