Creation of five-membered aromatic nitrogen ring opens door to new highly energetic rocket fuels and explosives
An aromatic ring of nitrogen atoms has been stably synthesised for the first time by researchers in China. This feat has allowed them to gain new information about this curious anion by applying techniques that were previously impossible. The research has significant fundamental interest, and might lead to the development of improved rocket fuels or explosives.
The dinitrogen triple bond is one of the strongest bonds in chemistry. This means nitrogen compounds such as azides can decompose extremely exothermically, making them useful high energy density materials. The proportion of nitrogen strongly influences the energy density of a material, so a pure nitrogen material would have obvious appeal. However, there are no neutral allotropes of nitrogen at standard temperature and pressure, and only two stable polynitrogen ions have previously been isolated.
A third possibility, the anion cyclo-N5–, has excited significant interest because of its theoretical kinetic stability. It was isolated in electrospray ionisation mass spectrometry experiments in 2002 by Karl Christe of the University of Southern California, US, and colleagues2 and was recently identified in solution,3 but had never existed in a stable compound. Attaching a benzene ring containing a strongly electron-donating group can stabilise the ion at low temperatures by conjugation between the two aromatic π-systems. However, isolating the free ion had eluded researchers because the C–N bond to the nitrogen ring is much stronger than the N–N bonds within it.
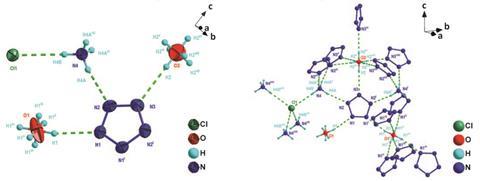
In the new research, Bingcheng Hu, Ming Lu and colleagues at Nanjing University of Science and Technology and elsewhere in China treated 3,5-dimethyl-4-hydroxyphenylpentazole with two reagents in solution: m-chloroperbenzoic acid to cleave the C–N bond and ferrous bisglycinate to stabilise the resulting cyclo-N5- anion. After purification, they isolated the air stable white solid (N5)6(H3O)3(NH4)4Cl. The free cyclo-N5– ions are stabilised by hydrogen bonds to both the H3O+ and NH4+ groups, allowing the compound to remain stable up to 117°C. The researchers performed several analyses not previously possible on the cyclo-N5- ion, measuring bond lengths and angles, all of which agreed with theoretical predictions.
The researchers are now seeking other, more energy dense cyclo-N5– compounds. ‘The molecule contains a lot of water ions, chloride ions and other non-energy functional groups,’ explain team members Bingcheng Hu and Ming Lu, ‘so the material of our article isn’t suitable for use as an explosive. The next step is to use metal cations and/or high-nitrogen cations to trap cyclo-N5-.’
Christopher Cummins of the Massachusetts Institute of Technology, US, says the paper ‘adds a new chapter to the story of inorganic aromaticity’. Christe believes huge challenges, and potentially huge opportunities, lie ahead. ‘To make a nitrogen allotrope is, for me, an enormous intellectual and scientific challenge,’ he says. ‘Defence organisations are very interested in seeing how much chemical energy they can put into materials… Polynitrogens clearly are the potential top performer of any material.’
References
1 C Zhang et al, Science, 2017, 355, 374 (DOI: 10.1126/science.aah3840)
2 A Vij et al, Angew. Chem., Int. Ed., 2002, 41, 3051 (DOI: 10.1002/1521-3773(20020816)41:16<3051::AID-ANIE3051>3.0.CO;2-T)
3 B Bazanov et al, Angew. Chem., Int. Ed., 2016, 55, 13233 (DOI: 10.1002/anie.201605400)





![A picture showing the molecular structure in the solid state of [(C2F5)(F3C)2CO]2](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/8/6/2/140862_Index-image-superfluorinated.jpg)
![Top view of the single-crystal X-ray diffraction structure of a dithienothiophene (DTT)-bridged [34]octaphyrin](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/3/6/2/132362_Bicyclic-Baird-type-aromaticity-fig-1-c.jpg)






No comments yet