Peptoids disrupt enveloped viruses’ lipid membranes as well as targeting fungi and bacteria
Peptoids – synthetic mimics of natural antimicrobial peptides – can inactivate a broad range of enveloped viruses by directly disrupting their lipid membranes. The molecules are also effective against certain bacteria and fungi, and get around some of the problems of developing peptides into drugs.
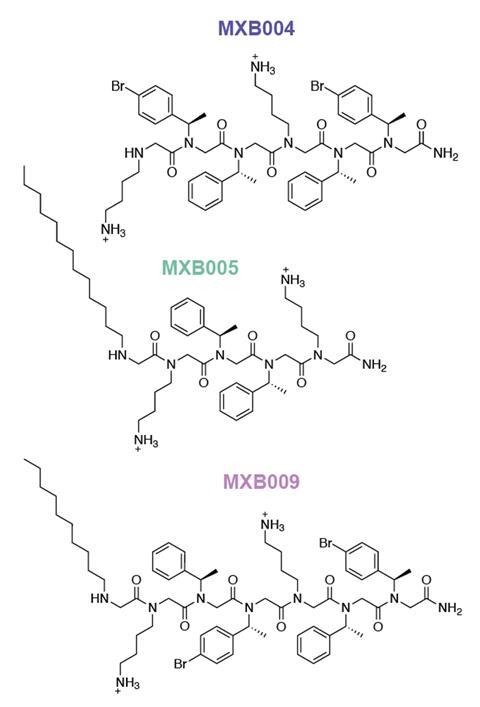
Attempts to harness the antimicrobial peptides that various organisms employ to defend themselves against pathogens have historically encountered various problems. ‘They are large, expensive to manufacture, and are rapidly degraded in the body,’ explains lead researcher Kent Kirshenbaum from New York University, US. Synthetic peptidomimetic compounds like N-alkylated glycine oligomers, more succinctly called peptoids, are more stable, smaller and have more favourable drug properties – while maintaining the antimicrobial actions, he explains.
Kirshenbaum’s team measured the antiviral activity of seven linear and cyclic peptoids against four RNA viruses with no current vaccine or treatment options: Zika, Rift Valley fever, chikungunya and coxsackie B3. They observed antiviral activity for the peptoids against the three enveloped viruses (which have an outer lipid membrane) but none against the non-enveloped coxsackie B3 virus.
To investigate the peptoids’ mechanism of action, the researchers prepared liposome particles with different membrane compositions and tested whether the peptoids would make them leak by disrupting the membrane. They found that liposomes containing phosphatidylserine were markedly sensitive to peptoids, while those containing only phosphatidylcholine were entirely insensitive. Sensitivity to peptoids also increased with phosphatidylserine content.
Because enveloped viruses, bacteria and fungi all display phosphatidylserine on their outer surfaces, whereas cells of the host organism generally don’t, peptoids could conceivably find use against a broad range of pathogens. Kirshenbaum is excited by the possibilities: ‘Imagine being able to stockpile the next generation of therapeutics capable of treating the next enveloped virus – before even identifying what that virus will be […] or being able to treat a complicated infection caused by a mixture of bacteria and fungi with one drug regimen – or being able to treat an infection of unknown origin without having to take the time to determine if it is caused by a bacteria or fungus.’
Kirshenbaum recognises that there will be a great deal of ‘gruelling work required to transform a promising molecule that has desirable activity in the lab into an actual drug that can work in the clinic’, with various potential problems still to overcome. Charles Chen, a visiting lecturer at King’s College London, UK, agrees. ‘Membrane-active peptides have been known to have off-target toxicity concerns,’ he says. ‘This is why many antimicrobial peptides cannot be used systemically and are only used for topical treatment against superficial infections. Therefore, the safety of antiviral peptoids needs to be carefully evaluated and studied.’
References
P M Tate et al, ACS Infect. Dis., 2023, 9, 1508 (DOI: 10.1021/acsinfecdis.3c00063)















No comments yet