Organic chemistry – Page 52
-
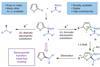 Research
ResearchCarbon–carbon couplings go 3D
Reaction extends 2010 Nobel prize winning Suzuki coupling and can be used to modify natural products
-
 Opinion
OpinionHow good do you want it?
Quality analysis is a very different ball game to a simple reaction check, says Chemjobber
-

-
 Podcast
PodcastNaphthalene
Once a fusty way of keeping moths out of clothes, Brian Clegg explains how naphthalene may have helped bring life to Earth
-
 Podcast
PodcastChemistry World podcast - May 2014
We speak to the ‘sultan of synthesis’, Phil Baran, and Peter James explains how labs can save cash on energy bills
-
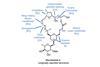 Opinion
OpinionMandelalide A
New reactions need to belong in a synthesis, says BRSM, not be forced in for show
-
 Podcast
PodcastDiphenyl oxalate
Nature has a range of ways to create bioluminescence. Not to be outdone, chemists create their own glow with diphenyl oxalate.
-

-

-
 Feature
FeatureThe sultan of synthesis
Phil Baran is spurring organic chemists to rethink how they make complex compounds, as Mark Peplow discovers
-
 Podcast
PodcastDichloromethane
It smells sweet, is potentially lethal and organic chemists rely on it: Emily James on dichloromethane
-
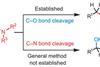
-
 Podcast
PodcastBatrachotoxin
Stephen Wallace introduces batrachotoxin, a deadly toxin that comes from a beautiful little frog
-

-
 Opinion
OpinionRinging the changes
A chemical manufacturing plant is a whole new world compared to the lab, as Chemjobber explains
-
 Opinion
OpinionTools of the trade
Derek Lowe wonders what the most life-changing instrument for organic chemists is, and what might be missing from the toolbox
-

-
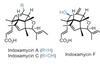 Opinion
OpinionIndoxamycins A, C and F
BRSM gets to the core of a divergent synthesis of this natural product family
-
 Podcast
PodcastAcetaldehyde
Brian Clegg meets a simple organic compound with a reputation for being something of a bruiser
-
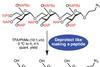 Research
ResearchDesigner esters for complex carbohydrates
Global deprotection in a single step streamlines oligosaccharide synthesis