Meera Senthilingam
This week, beware at the bar as Brian Clegg has some revelations.
Brian Clegg
There was a time when a member of the criminal fraternity could be accused of slipping someone a 'Mickey Finn.' This seems to have been named for a Chicago bartender who joined the line of infamous hosts who have incapacitated their customers to rob them. Michael Finn's name went down in history after he used the rather subtle means of giving his customers knock-out drops - rather than a knock-out blow - before robbing them. And in all probability the drops he used were chloral hydrate.
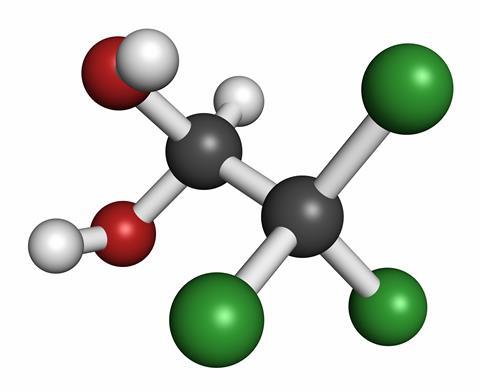
The 'chloral' part of this relatively simple organic compound is trichloroethanal, also known as trichloroacetaldehyde. One of the ethane skeleton's carbon atoms has three chlorine atoms attached; the other has an oxygen and a hydrogen atom, making it an aldehyde. To turn it into chloral hydrate, a water molecule is added, giving it a pair of hydroxyl groups.
Although, in principle, chloral hydrate can easily be made from chloral by adding water, in practice chloral itself is made from chloral hydrate, by treatment with sulfuric acid. Chloral is not much use in its own right, but is a starting point in the manufacture of DDT and can also be used to produce chloroform, though this is usually done by reacting chlorine with methane. To make chloral hydrate in industry, chlorine is reacted with ethanol in the presence of an acid.
Chloral hydrate was first produced in 1832 by the great German chemist who first discovered nitrogen's role in fertilizers and made great strides in early organic chemistry, Justus von Liebig. At the time it was considered little more than another member of his prized collection of new organic compounds, but by the late 1860s it was being used as a sedative and its relative simplicity to manufacture made it popular for both legal and more dubious purposes.

Medically, chloral hydrate acts both as a sedative to calm patients and, with appropriate doses, as a hypnotic, putting the patient to sleep. These effects mean that it has been used in the past to give a sedating effect before surgery, though it has now been replaced by safer and better controlled substances. It is still occasionally prescribed to treat insomnia, to help sufferers relax sufficiently to achieve sleep. Though it can be provided as chloral hydrate in liquid form, for insomnia it also turns up in tablets in a precursor form, chloral betaine.
It was the hypnotic application of chloral hydrate, typically in an alcohol solution, that got it the reputation of being a safe and reliable form of knockout drops - the infamous Mickey Finn. In reality it was neither safe nor reliable. There is no simple mechanism, whether it involves a cosh or a chemical, that can render someone unconscious safely. Anaesthesia is a complex business that needs careful monitoring. Although chloral hydrate certainly can be used to knock someone out - and was sometimes employed this way medically before better replacements were introduced - it needs a carefully handled dosage for the individual. Getting it wrong could result in nothing more than drowsiness or, at the extreme of an overdose, in nausea, convulsions and coma.
Medically, chloral hydrate remains in a kind of legal twilight. It is an unapproved drug that is sometimes still used, but that has not had sufficient clinical trials to determine how to use it appropriately (in part because it is too easy and cheap to manufacture to make this worthwhile for a pharmacological company). In some countries, like the US, it is a controlled drug, but not, for example, in the UK - though it does require a prescription to buy it. Aside from occasionally being prescribed for insomnia, its formal medical use has pretty well dried up, because there is usually a better and safer alternative. It is, however, sometimes still used in association with EEG tests as it has less influence on some brain functions than most sedatives.
The drug influences the brain by increasing the effectiveness of the receptors for the neurotransmitter gamma-aminobutyric acid. This amino acid is an important inhibitory neurotransmitter and there is a whole range of medicines that act to provide relaxation and reduced anxiety by either increasing its production or the receptiveness of the neurotransmitter. As often is the case with such compounds, the effectiveness can reduce with repeated use and chloral hydrate can become addictive.

We've already seen that chloral hydrate is a stepping stone on the way to chloral, but it can also be used to form isatin, an aromatic compound that is at the heart of a blue dye. It also plays a couple of roles in the production of microscope slides, both acting as a clearing agent for plant material in a mix with lactic acid, and combined with glycerol and gum arabic to form a medium for mounting specimens on microscope slides that are to be kept for some time. It's not exactly a giant of industrial chemistry, but neither is it without benefit.
Chloral hydrate has its positive uses, then - but it's hard not to feel this is a bit like pointing out that a professional criminal does charity work in his spare time. Like it or not, chloral hydrate's claim to fame will always be helping the likes of Michael Finn relieve his customers of their consciousness and their cash.
Meera Senthilingam
So uses in medicine but also in manipulation. Science writer Brian Clegg there with the Jekyll and Hyde chemistry of chloral hydrate. Next week, we go in search of drugs and dig deep beneath the soil.
Simon Cotton
After the success of penicillin, people searched for other antibiotics in nature. In the 1940s, they discovered the first of the tetracycline drugs, made by soil fungi for their defence. In 1953, a new antibiotic, vancomycin, was discovered in a soil sample from Borneo. It was found to be effective against resistant strains of bacteria, and came to be regarded as the drug of last resort, reserved for infections resulting from bacteria that are resistant to all other antibiotics.
Meera Senthilingam
And you can discover the chemistry enabling this ability of vancomycin to treat all by joining Simon Cotton in next week's Chemistry in it's element. Until then, thank you for listening. I'm Meera Senthilingam.












No comments yet