A catalyst that reacts only with aryl iodides, spurning bromides and chlorides
As funding for total synthesis has dwindled, the number of graduates combining extensive practical knowhow and outstanding retrosynthetic vision has declined, to the dismay of many. But does it really matter? Computational retrosynthesis programs that also select optimal reaction conditions are in the offing, and like it or not, computers will soon be good enough to replace a significant portion of human input in synthesis design. So what next for organic chemists? We are clearly not out of a job: great practical skills and chemical knowledge are still crucial to sense-checking and executing any synthesis a computer spits out, but these new tools give us the opportunity to redirect our energies.
If synthesis planning software lives up to its promise, efficient syntheses won’t be limited by individual human knowledge and retrosynthetic foresight, but by known reactions and available building blocks. As computers can’t (yet?) independently invent new reactions and chemists have little influence on which molecules they can buy, it is clear what we need to be doing. Synthesis is a long way from having an optimal toolbox of reactions. One obvious (and still distant) ideal is the ability to selectively react any functional group in the presence of any other. There is a lot of room for invention here, and uniquely selective reactions are highly sought after. This is the focus for Franziska Schoenebeck and her group at the University of Aachen, Germany, who have discovered a trinuclear palladium cation that selectivity couples Grignard reagents with aryl iodides in the presence of both aryl chlorides and aryl bromides; selectivity that is very hard to achieve.
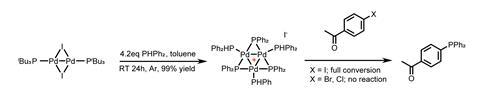
Reacting a previously reported dimeric Pd(i) catalyst with diphenylphosphine forms an air- and moisture-stable trinuclear palladium cation, in 99% yield. The x-ray structure reveals a triangular arrangement of the palladium atoms and computational studies suggest that the complex is an aromatic, singlet closed shell cation! The bench-stable catalyst’s reactivity with three analogous aryl halides (figure 1) was evaluated and the team surprisingly found that it only reacts with an aryl iodide, not the bromide or chloride.
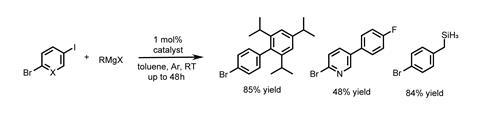
Looking to exploit this unusual reactivity profile, the researchers investigated cross-coupling polyhalogenated (I and Br) arenes with Grignard reagents. Reacting 1-bromo, 3-iodobenzene with phenylmagnesium bromide saw exclusive reaction of the aryl iodide, with the bromide remaining intact (Figure 2). The reaction has a rather impressive scope, with complete selectivity for reaction at iodides over bromides in each instance.
A range of aryl and alkyl Grignard reagents gave typically very good yields. Even the sterically hindered 2,4,6-triisopropylphenylmagnesium bromide and ortho-substituted iodoarenes proved no major problem, although combining the two led to reduced yields. The team has yet to propose a rationale for this, but I wonder if reductive elimination of two aryls from adjacent Pd atoms (rather than the same Pd atom as with mononuclear catalysts) reduces the influence of the steric bulk. Heterocyclic iodopyridines and pyrimidines also reacted well, even maintaining selectivity over bromides activated by neighbouring nitrogen atoms.
Probing the essentially unexplored realm of reaction mechanisms with trinuclear metal catalysts, the team took a dual experimental and computational approach. Experimentally, the lack of Br vs I selectivity for reactions using a number of mono- and diatomic Pd(0), (i), and (ii) species suggests that the trimeric cation is the active catalyst, rather than acting as a reservoir for another catalytic species. The team also ruled out palladium nanoparticles as a catalyst, having observed an unselective reaction with a prepared sample, although this definitely needs digging into in more detail as nanoparticles are by no means a homogeneous class. Some simple filtration tests and kinetic studies would go a long way to support these conclusions. DFT calculations, at least to a computationally naive organic chemist such as myself, demonstrate a very clear hypothesis for the selectivity. The transition state energies for oxidative addition of the trimer and the aryl chloride, bromide and iodide show a clear parallel with bond strength (Ar–Cl > Ar–Br > Ar–I). A significant difference of 8.1 kcal/mol between the aryl bromide and iodide transition states could readily account for the impressive selectivity.
With the rise of the machines, chemists may fear for their professional futures. But they really shouldn’t. We should be proud that we have made major steps towards increasingly trivialising aspects of synthetic planning and take the opportunities that this affords us: more time to push boundaries, solve problems and innovate. Chemists will do what they have always done: work at the cutting edge of science.
References
C J Diehl et al, Angew. Chem. Int. Ed., 2019, 58, 211 (DOI: 10.1002/anie.201811380)








No comments yet