The Nobel prize that got binned
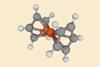
Ferrocene is a classic example of a discovery that was dismissed as a failed experiment
The discoveries that are recognised by the chemistry Nobel prize often exhibit certain features: inspiration, intelligence, rivalry, disputes about credit and no small amount of sheer luck. I would add ‘the ability to recognise something important when you see it’ to that list.
