Katia Moskvitch finds out about the latest techniques to determining a molecule’s chirality
What do oranges and lemons have in common, but which provides their main difference? It’s not their taste, shape or colour. The molecules responsible for their distinctive smells have no chemical differences and nearly identical formulas. Both contain limonene, but those in lemons are the mirror image of those in oranges; like left and right hands, they are identical and yet different. This handedness of molecules – containing the same atoms but arranged in two mirror-image configurations – is called chirality.
What may be a curiosity for lovers of citrus fruit is of potentially deadly importance to the pharmaceutical industry. The ‘wrong-handed’ version of a molecule in a drug can work totally differently in the body. So scientists are keen to discover which way a molecule is orientated, and are working currently on doing that more effectively.
The word chirality comes from the Greek word cheir, which means ‘hand,’ because hands are the most widely recognised example of chirality – they are not identical when superimposed on their mirror image. Many, but not all, naturally occurring chemicals exhibit chirality, and the non-superimposable mirror images of a chiral molecule are known as ‘enantiomers’ or optical isomers.

Most building blocks of nature, for example sugars and amino acids – key components of proteins – are chiral. But life on Earth exclusively uses right-handed sugars and left-handed amino acids. This fact has puzzled scientists, according to Jan Labuta, a chemist at the National Institute for Materials Science in Japan. Many believe ‘that the choice of chirality was accidental and if the choice was opposite, life could be the same just using the opposite chirality molecules’, he says. ‘In the early stage of evolution, it just happened that one-type-chirality building blocks of life were available in excess.’ After a biological system’s death, however, amino acids start to turn into their ‘dextro’, or right-handed, versions, because of protein degradation. Archaeologists use this fact to date organic material: by comparing the proportion of left- to right-handed molecules they know how long something has been dead.
It came from outer space

Researchers now think that nature’s specific handedness may have originated in space, with the first molecular structures of life forming in the interstellar medium, before hitching a ride on meteorites and comets to the young Earth. ‘Molecules in a certain region of space could be exposed to extremes of circularly polarised radiation from neutron stars that could produce an excess of one handedness of chiral molecules more easily than here on Earth,’ says Laurence Nafie of Syracuse University in the US.
We may soon be able to verify this theory, once the Rosetta spacecraft has landed on comet Churyumov–Gerasimenko in November 2014, to study the composition of its nucleus. In anticipation of the landing, a team led by Meierhenrich simulated cometary ice and analysed its composition. ‘We produced several micrograms of an artificial comet in the laboratory and surprisingly identified 26 amino acids, many of them chiral,’ says Meierhenrich.
He says the molecules were not limited to amino acids, but included chiral hydrocarbons, chiral alcohols and diols, along with many other chiral compounds. ‘Designing and developing the chirality investigations of the Rosetta mission, we spent 15 years optimising analytical techniques to detect and quantify chiral molecules. Without Rosetta, this advancement would certainly not have been realised.’
Helping hands
Here’s the big problem: although these molecules are nearly identical, our cells respond in the desired way to only one of the mirror configurations, in the same way only a right hand can fit comfortably in a right glove.
This means that chirality is very important for drugs. ‘90% of today’s drugs are chiral and 90% of those are sold as racemic mixtures – mixtures of both enantiomers,’ says Uwe Meierhenrich, a physical chemist at the University of Nice in France. ‘As only one of the two enantiomers provides the desired pharmacological effect, the other (wrong) enantiomer should not necessarily be taken up by the consumer.’
Understanding and controlling chirality has allowed the pharmaceutical industry to create drugs such as beta-blockers, used to treat heart-related disorders and high blood pressure. Citalopram, an antidepressant, is produced as a racemic mixture, but only the (S)-(+) enantiomer is responsible for its beneficial effects. The drug d-penicillamine can treat rheumatoid arthritis, but l-penicillamine is toxic. For years, scientists have been developing ways to choose the specific shape of a molecule and synthesise only that chiral version. The research has helped develop various antibiotics, anti-inflammatory drugs and heart medicines.
90% of today’s drugs are chiral
Uwe Meierhenrich
But effective, correct and rapid determination of chirality in a mixture is still tricky. Now scientists are pushing new ways to detect which chiral form of a molecule is present in a sample, which could be the basis of huge pharmaceutical breakthroughs.
Distinguishing chiral molecules is obviously important, but difficult. Louis Pasteur could do it by spotting optical rotation caused by chiral molecules in his beaker. ‘But if he had a mixture of right-handed X, left-handed Y, racemic Z, and so on, he couldn’t pick that apart,’ says physicist David Patterson of Harvard University in Cambridge, US, an expert on detecting chirality. ‘Complex mixtures of chiral molecules are extremely common – for example, most chemical reactions leave some sort of mixture, and our bodies certainly contain extremely complex mixtures.’ Optical rotation is still used today, but only for basic characterisation of chirality.
Separating out

The most common method used today to separate enantiomers is liquid or gas chiral chromatography. Once enantiomers are separated, one can determine their respective absolute configuration – or handedness – by methods such as circular dichroism, vibrational circular dichroism, optical rotation, nuclear magnetic resonance (NMR) and x-ray crystallography. Only the latter, though, can determine the absolute configuration directly, says chemist Juergen Stohner of Zürich University of Applied Sciences in Switzerland, ‘whereas all other methods need support by reference compounds of known handedness or computer simulations’.
But all these methods have limitations. In chromatography, ‘the separation works because of different interactions between each enantiomer and chiral selector’, says Labuta. This method is very sensitive and relies on very carefully controlled surface chemistry, as the chemical to be analysed elutes down a narrow capillary – but it is also very susceptible to external parameters. Chromatography cannot establish a standard because it ‘must be tweaked for each individual chemical to be studied – companies that make the chiral columns are getting better and better at it, but it’s still not a «plug and play‰ solution’, says Patterson. ‘In the hands of a skilled operator, chromatography can give very good results, but what you want in a standard is something that acts the same, every single time – ideally something that you could describe to somebody across the world, and when she does it she gets the same answer you did.’
Circular dichroism (CD) – differential absorption of left and right circularly polarised light – is a popular technique as well, but it can only detect chirality in fairly simple systems. It is often used in research to ‘quantify’ chirality – for chirality transfer, chiral complex structures such as protein tertiary structure, and so on – since it touches the fundamentals of chirality, says Labuta. ‘However, it lacks required precision,’ he adds.
Vibrational circular dichroism (VCD) is much more precise, according to Nafie. ‘It’s the extension of CD, measured in the visible and UV regions of the spectrum, where electronic transitions occur, to the infrared region where vibrational transitions occur,’ he says. The infrared (IR) ‘fingerprint’ region has many more of a molecule’s absorption bands than the UV region does. This means that VCD has an enhanced ability to sense chirality and distinguish enantiomers without chemically modifying the molecule or adding a discriminating agent, Nafie explains. ‘VCD is now used by all major pharmaceutical companies, and many minor ones, for determination of absolute configuration – which of two enantiomers is present,’ he adds.
Another method of chirality detection and analysis emerging for broader use is Raman optical activity (ROA). This measures the difference in Raman scattering between right and left circularly polarised light in the scattered radiation. ROA is complementary to VCD, and its strength is in its sensitivity to the structure of proteins in solution under various environments, says Nafie. ‘This is vital information for the biopharmaceutical industry, where many new drugs are based on protein molecules.’
New tricks
But new, more exact methods are still needed, according to Labuta. Together with his colleagues, he has recently reported a new NMR technique1 that uses the concepts of supramolecular chemistry where non-covalent interactions between molecules such as hydrogen bonding, electrostatic or van der Waals forces play important roles in the properties and shape of a molecular assembly.
This approach gives rise to new functionality in the assembly, which could not be obtained from its individual building blocks. The new NMR technique applies this strategy to form host–guest complexes between two species – a detector molecule and a chiral sample. ‘The detector is an achiral porphyrin derivative – structurally similar to the oxygen trapping centre of haemoglobin in blood – which can interact with a whole variety of chiral substances,’ Labuta says.
When a chiral sample is mixed in solution with the detector, the chiral information is transferred via non-covalent interactions from the sample to the detector and observed in its NMR spectrum. ‘The achirality of the detector leads to potential niche applications, for instance in situ monitoring of racemisation or asymmetric reactions,’ says Labuta. ‘Another advantage is that a lot of possible chiral molecules can be analysed using a single detector.’ The method may find applications in specific tasks where standard NMR techniques don’t work because they might bias the result of enantiomeric purity analysis.
Another new method is being developed by Patterson together with John Doyle of Harvard and Melanie Schnell of the Centre for Free-Electron Laser Science in Hamburg, Germany. The technique2 uses quantum physics and microwave spectroscopy – the ability of microwaves to rotate a polar molecule – to determine whether a gas phase molecule held in an electric field is left- or right-handed (see Microwaves show their hand).
We can tease enantiomers apart without chemically separating the mixture
David Patterson
The team used gas-phase samples of (S)- and (R)-1,2-propanediol and a racemic mixture of the two. They first cooled the samples to 7K to reduce the number of occupied rotational states and bathed the mixture in an electric field that interacted with the electric dipole of the sample compound, with opposite signs for each enantiomer. This difference showed up in the phase of emitted microwave radiation.
‘Imagine a ball with bumps on it. If I wiggle it one way, the bumps on the side will wiggle in the opposite way,’ says Doyle. ‘So if the ball is a molecule, we were able to wiggle it one way by applying microwave radiation, and watch the bumps “move”. The ball wiggles in such a way that it emits microwave radiation, and we can tell whether it is a left-handed ball or a right-handed ball, depending on the kind of radiation it emits.’
Patterson hopes his group’s method will bring two new features to the many techniques already available. Since it relies on narrow resonances – very specific frequencies are used for each species – it is inherently compatible with mixtures. ‘Our method can in principle tease [molecules] apart without chemically separating the mixture,’ he says.
Vibrational circular dichroism can do the same but is limited to a mixture of two or three components. ‘We hope to be able to do much more complex mixtures,’ says Patterson, adding that his team’s method is at the moment the most mixture-compatible technique available.
‘Doyle’s method is promising for fast analysis of samples with many types of chiral molecules. This might be a breakthrough if it will be developed to the point where it can be used easily in medical analysis,’ says Labuta.
Explosive advance
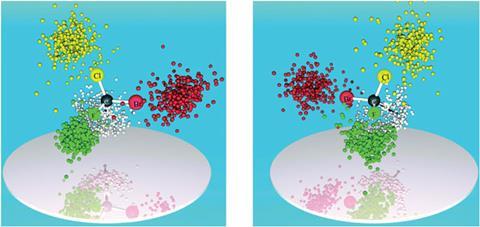
Finally, Stohner and colleagues have recently developed a direct method3 that, they say, is better than x-ray crystallography (see Unravelling stereochemistry via mass spectrometry). They used Coulomb explosion imaging to determine the structure of chiral molecules in the gas phase. The scientists first shot a laser into the molecules, stripping them of electrons and making them explode into tiny pieces. These pieces then smashed into a detector. By knowing a piece’s mass, flight time to the detector and position when it hit the detector, it was possible to calculate its momentum. This allowed the team to find out the fragment’s original position around the central carbon atom.
The technique became feasible because high-power lasers with high repetition rates are now commercially available. Today’s high-performance detectors and fast data acquisition are also key for those experiments. There is potential to complement or even replace x-ray crystallography once miniaturised for more routine applications. The applicability to large molecules, such as those developed for drugs, however, still needs to be explored.
‘In contrast to x-ray crystallography, no single crystals are needed, which is a great advantage since not every substance crystallises and the method’s applicability is therefore much broader,’ says Stohner. ‘One can also «see‰ isotopes in natural abundance and might study isotopically chiral compounds.’ Sensitivity towards both light and heavy atoms is another advantage over x-rays.
At the moment, there is still not one best, simple method for quickly and efficiently detecting chirality. All chiroptical methods (VCD, CD, ROA and OR) are mutually complementary, says Nafie. ‘They all have the advantage over x-ray crystallography in that they do not require a single crystal of sufficient size and quality for analysis; they only need a solution phase spectrum. VCD is the easiest and most reliable, next is CD, ROA is accurate but the calculations take longer and the instrumentation is more expensive than VCD and hence is used less frequently so far, and finally OR, least reliable and seldom used.’
But, says Patterson, hopefully one day it will be possible to ‘make a machine in which you inject a mixture, hit a button, and it beeps and thinks and tells you everything that’s in there – including the chirality’.
Katia Moskvitch is a science writer based in London, UK
References
1 J Labuta et al, Nat. Commun., 2013, 4, 2188 (DOI: 10.1038/ncomms3188)
2 D Patterson, M Schnell and J M Doyle, Nature, 2013, 497, 475 (DOI: 10.1038/nature12150)
3 M Pitzer et al, Science, 2013, 341, 1096 (DOI: 10.1126/science.1240362)













No comments yet