A modified bacterial enzyme can produce the rare sugar nigerose from table sugar. This could provide a cheap and convenient route to make enough nigerose to explore potential applications in the food and pharmaceutical industries.
Nigerose is found naturally in trace amounts in Japanese sake rice wine. It comprises two glucose molecules connected by an oxygen link between the C1 hydroxyl of one and the C3 hydroxyl of the other. Glucose’s structure means it can combine in 11 different ways, some of which are more common in nature, such as a 1,4-linkage found in starch.
Others like the 1,3-link in nigerose are much rarer. This means digestive enzymes are not evolved to degrade them, and so these sugars may behave and taste differently to more common sugars. For example, 1,2-linked kojibiose is a low-calorie disaccharide found in honey that may aid against tooth decay, unlike common sugars.2
Previous research had shown a sucrose phosphorylase from Bifidobacterium adolescentis could synthesise nigerose when using DMSO as a cosolvent.3 However, the cost of using DMSO renders the method unsuitable for industry. Tom Desmet and his team at Ghent University, Belgium, have mutated this enzyme to catalyse formation of nigerose on a multi-gram scale in aqueous solution, eliminating the need for DMSO.
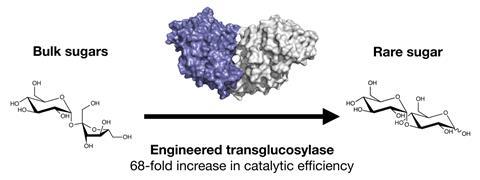
To create the mutant enzyme, Desmet’s team targeted two specific parts of the protein sequence that affect the shape of the active site. After breaking down the original sucrose disaccharide, the enzyme activates the resulting glucose ready for selective attack from a further glucose molecule, forming nigerose.
Desmet stresses that this is fundamental research, so they do not yet know whether these sugars could end up being used. Non-digestible sugars are useful in the food industry as sweeteners, for example, but they may find further use as drug scaffolds or in pesticide development.
Alison Narayan from the University of Michigan, US, who develops enzymatic methods to solve synthetic problems, admires the beauty of this biosynthetic reaction. ‘Now, there’s a tool to make this sugar efficiently with no protecting groups, enzymatically, at scale,’ she says. ‘That sugar becomes something that people can think about using … this work really shows a concept that I think a lot of synthetic chemists benefit from seeing.’
The team hopes that elaborating this approach and further engineering could enable the synthesis of all 11 glucose disaccharide variants, allowing for broader industrial testing. Desmet says the work is ‘just scratching the surface of this bigger diversity that is available. At some point you have to just make the jump and see what else is out there.’
References
1 J Franceus et al, Chem. Commun., 2019, 55, 4531 (DOI: 10.1039/c9cc01587f)
2 T Verhaeghe et al, Chem. Commun. 2016, 52, 3687 (DOI: 10.1039/c5cc09940d)
3 M Kraus et al, Chem. Commun., 2016, 52, 4625 (DOI: 10.1039/c6cc00934d)














No comments yet