UK researchers have produced a workflow for chemists and chemical engineers to follow when designing continuous crystallisation processes. The pathway will help users navigate the black art of crystallisation to manufacture pharmaceuticals with greater precision.
Crystals are the building blocks of most medicines, giving purity and stability to their active ingredients so that they can be formulated into tablets. However, crystallisation is a highly complex process, and factors such as solvent choice, temperature and pressure can have massive effects on the purity and properties of the final product. Continuous crystallisation allows researchers to make large quantities of a drug by extending the duration of the process, or by running multiple parallel processes. It can have huge benefits for pharmaceuticals, giving more consistent final products, and allowing researchers to make large volumes of material on smaller equipment, reducing cost.
Choosing a method for crystallisation traditionally relies on the knowledge and experience of the researcher so can involve many rounds of trial and error, wasting time and material. Now, Alastair Florence from the EPSRC Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation (CMAC) and his team have developed a workflow that allows users to work out the best crystallisation conditions for their product.
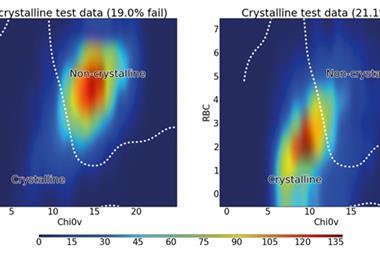
The workflow outputs a continuous process that can deliver required quantities of crystalline product with precise properties, on demand. It works by dividing the crystallisation process into automated steps coupled to modelling tools. It considers solubility and solvent selection, suitable analytical methods and the crystallisation platform. A decision point follows each step, where the system analyses relevant data then advises the user how to proceed. As the workflow is used for different compounds, the team will be able to generate data sets so that they can use machine learning to further develop it.
An initial run on the workflow took two researchers 12 months and 5kg of material to identify the final process, but a more recent run with a single researcher took 4 months and 1kg of material. Florence explains that CMAC’s aim is to reduce this to 10 days and 10g, and the team is confident they can reach this target. The team carried out a case study on paracetamol to prove their concept worked, and produced 6.5kg of the drug with controlled particle size distribution. They have also applied the workflow to a number of other drugs with good results.
Mark Roelands, an expert in continuous crystallisation at the Netherlands Organisation for Applied Scientific Research, says the workflow ‘gives a well-structured approach to crystallisation process development, with options to study specific aspects deeper where needed.’
Florence notes that fellow researchers are often very keen to use such data-driven tools in their research, particularly where first principles models may not be reliable. ‘Data is increasingly important in the digital industrial age, and it is important that researchers at all stages in their careers recognise the value in recording, storing, and sharing their data,’ he adds.
Roelands sums up the impact of the work by saying: ‘Crystallisation is often considered more of an art than a science … The workflow takes some of the art out and introduces some solid science in this area.’
References
This article is open access
C J Brown et al, Mol. Syst. Des. Eng., 2018, DOI: 10.1039/c7me00096k





















No comments yet