Salivary peptide fragments from a leech, a tsetse fly and a rat flea have been combined to create a synthetic protein that clamps onto thrombin, the key enzyme in blood clotting, with remarkable strength. Developed by Richard Payne and Joshua Maxwell at the University of Sydney, Australia, and colleagues, they believe their hybrid molecule could inspire alternative design strategies for new anticoagulants.
Cardiovascular disease remains the world’s leading cause of death and is largely driven by thrombotic events such as strokes and heart attacks. Anticoagulants work by inhibiting thrombin or disrupting other steps in the coagulation cascade. However, they come with risks and limitations – particularly during surgery and in preventing coronary artery disease – due to their narrow therapeutic window and potential to cause bleeding. These challenges are driving efforts to develop safer and more effective alternatives.
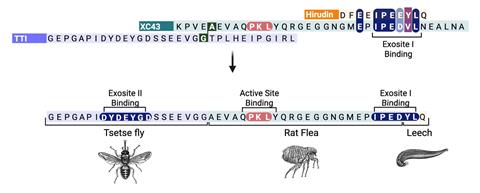
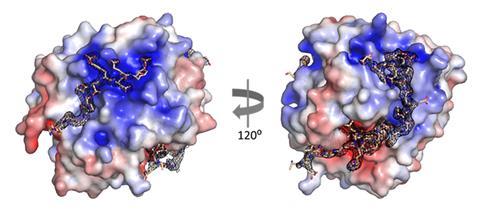
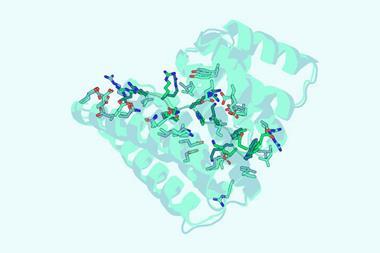
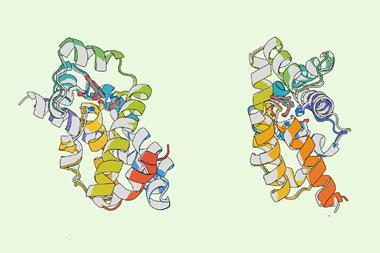
XChimera, the molecule developed by Payne, Maxwell and colleagues binds thrombin at three distinct sites: its active site and two exosites that regulate clotting and platelet activation. This multi-site engagement achieves femtomolar inhibitory binding with thrombin that is orders of magnitude tighter than standard thrombin inhibitors.
‘Even though we very carefully designed the molecule from the three component anticoagulants from leech, fly and flea peptides, we expected the hybridisation to induce some changes in the way each component bound to thrombin,’ says Maxwell. ‘One of the big surprises was just how well XChimera mirrored the binding of the individual leech, fly and flea molecules from which it is derived,’ adds Payne.
To assemble the hybrid peptide, the team combined solid-phase peptide synthesis, chemical ligation and post-synthetic sulfation. They then used x-ray crystallography to show that all three thrombin sites were engaged simultaneously. Complementary biochemical and clotting assays confirmed the hybrid protein was a potent thrombin inhibitor and able to prevent platelet aggregation.
‘This study demonstrates not only an inhibitor of extraordinary potency but also one with a novel activity profile,’ comments Nicolas Winssinger, a bio-organic chemist from the University of Geneva in Switzerland. ‘It inhibits both thrombin’s proteolytic activity and platelet aggregation. This dual action offers exciting potential for the development of safer and more effective anticoagulants.’
In mouse models, XChimera significantly delayed clot formation and reduced thrombus size, without showing immediate signs of toxicity. Still, Maxwell and Payne caution that human trials remain a long way off.
‘I think the chemistry is elegant. It’s a really cool idea. They show very nicely that they can make a very tight-binding thrombin inhibitor,’ comments Jeffrey Weitz, a physician and blood coagulation expert at McMaster University in Canada. ‘But the major problem is it’s essentially a potent irreversible inhibitor of thrombin, and because of thrombin’s importance in haemostasis, that could pose safety issues.’
Beyond anticoagulation, Payne says the strategy of bioactive peptide hybridisation could be extended to other therapeutic areas where disrupting multiple protein–protein interaction sites within a single protein target is desirable.













No comments yet