Ben Valsler
This week, how do we decide what drugs to stockpile in case of an emergency? Chris Chapman looks at tamiflu, and the pandemic that pushed us to question our decisions.
Chris Chapman
In 2009 you could have been forgiven for thinking the world was coming to an end. The much-feted flu pandemic had finally arrived, and H1N1 – swine flu – was set to wipe humanity off the map. In hastily drawn up plans, the UK government began issuing affected patients with Oseltamivir – Tamiflu – as part of its contingency.
Fortunately, swine flu turned out to be a relatively tame pandemic; although at its height around 110,000 people in the UK were infected each week, just 214 deaths were recorded. But although the infection had been mild in the majority of cases, the aftermath was brutal for open science and government decision-making.
After the emergence of bird flu in 2005, the UK government began stockpiling two treatments – Tamiflu and the related zanamivir, or Relenza. This stockpile came in at a cost of around half a billion pounds – around 5% of the National Health Service’s entire annual primary care drugs budget, and roughly enough for 40 million doses. The US government spent some $1.3 billion. The World Health Organisation added oseltamivir to its list of essential medicines.
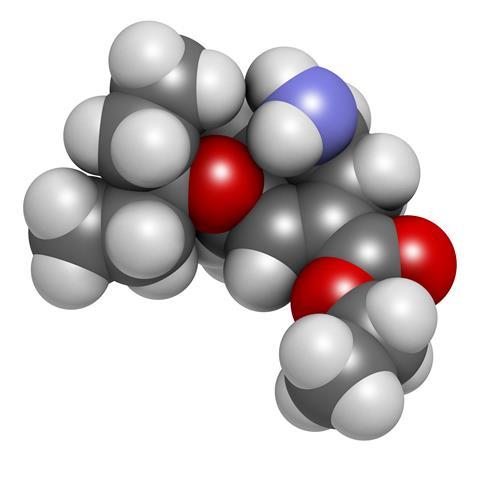
Of course, oseltamivir was never meant to cure flu, but rather shorten the duration and severity of symptoms. Taken as a capsule of oseltamivir phosphate, it is a prodrug, undergoing ester hydrolysis in the liver to oseltamivir carboxylate. The supposed mechanism is that it inhibits influenza virus neuraminidase – an enzyme required to release viral particles from infected cells – which prevents the infection from spreading. All of this comes at the price of side effects, most commonly headache and nausea.
All of this sounds eminently sensible in practice. If a drug is an effective way to reduce illness, then in a pandemic it can save lives, reduce hospital admissions, and keep the country going.

The problem is that it might not have been the best option. During the swine flu pandemic, the UK and Australian governments asked the Cochrane Collaboration – an independent global network that aims to create credible health information – to update its review on the evidence behind these inhibitors; it was important to assess if the stockpile had been a worthwhile use of the public purse.
At first, Cochrane didn’t envisage a problem: their review had been updated in 2008, and stated they didn’t believe the changes would require substantial effort. However, a cursory look at the evidence behind the drugs came up with a substantial stumbling block. One of the most extensively cited pieces of evidence for oseltamivir was a 2003 meta-analysis, published in the Archives of Internal Medicine. However, the Cochrane team were unable to access complete data. Eight of the 10 trials analysed in the paper had not been published in peer-reviewed journals.
The answer, Cochrane found, was to modify their approach. The team stopped focusing on data reported in biomedical journals, and moved to clinical study reports and regulatory documents. As of its latest review, the Cochrane team had looked at 107 clinical study reports for oseltamivir and zanamivir to get a true feeling of its effect; after discarding trials they found to be at risk of bias, they used data from 46 trials – 20 of which were for oseltamivir. It found that oseltamivir did indeed reduce the time taken to relieve symptoms by about 17 hours, although there wasn’t any strong evidence it worked in children with asthma. There was no evidence it affected hospitalisations. Overall, the authors concluded that yes, both drugs shortened the duration of symptoms. But then, so does paracetamol. And, perhaps most interesting of all, the mechanism of action was questioned too, with Cochrane reporting that ‘statements made on the capacity of oseltamivir to interrupt viral transmission and reduce complications are not supported by any data we have been able to access. The mechanism of action proposed by the producers (influenza virus-specific) does not fit the clinical evidence which suggests a multi-system and central action.’
This twisting tale has raised serious concerns about whether there was really a need to spend billions globally stockpiling the drugs, when the evidence suggests that they’d reduce the length of illness by only a few hours. But it also highlighted a bigger problem: clinical trial data.
Lack of access to clinical trial data was, of course, not a new problem, or one limited to oseltamivir. Yet the Tamiflu saga – brought to light by Cochrane and the British Medical Journal – is one of the most high profile cases where a closer inspection of data raised serious concerns. Oseltamivir has become a poster child of the All Trials movement, which continues to gather momentum and may yet lead to every clinical trial being registered and reporting its findings in full. Should this succeed, and scientists and clinicians have access to the full picture about a drug, the Tamiflu saga will be remembered: it could be that those drug stockpiles for a flu pandemic were money well spent.
Ben Valsler
Chris Chapman, Chemistry World’s opinion editor, on the serious questions that remain around transparency of clinical trial data. Next week, Raychelle Burks finds how it’s not always the dangers you expect that cause problems…
Raychelle Burks
For one American man in the late 1990s, too much cola resulted in a visit to the emergency department of his local hospital. He complained of headache, fatigue, confusion, and an inability to control bodily movements (called ataxia). But it wasn’t the sugar or caffeine that landed him in emergency care, it was the bromide.
Ben Valsler
Find out how soft drinks can land you in hospital in next week’s Chemistry in its Element. Until then, you can catch up with our monthly magazine and book club podcasts at rsc.org/chemistryworld, and send any questions or comments you have to chemistryworld@rsc.org or tweet @ChemistryWorld. I’m Ben Valsler, thanks for listening.










No comments yet