Evidence supports new theory underpinning dark blue to pink-orange transformation
A multinational team of scientists has proposed a new explanation for the striking colour change observed when a lobster is cooked.1
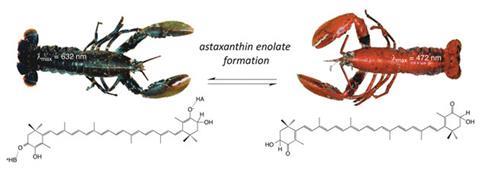
The dark blue to pink-orange transformation revolves around astaxanthin, a carotenoid, and its interaction with a protein called crustacyanin that lobsters accumulate by feeding on plankton. Astaxanthin complexing with crustacyanin endows lobsters with their dark blue camouflage, but crustacyanin denatures when cooked, releasing astaxanthin, which is orange in its unbound form. While this has been known to cause the colour change for some time, the actual mechanism has been the subject of debate.
The question is what is happening to this molecule in its bound and unbound states that causes such a pronounced change in its colour? Several theories have been suggested, involving coplanarity, polarisation and exciton coupling, however recent studies have found that these effects cannot account for more than 30% of the observed colour shift.
For John Helliwell at the University of Manchester, UK, this phenomenon has been a long-running research theme – his group obtained the original x-ray structure showing the binding of astaxanthin in 2002.2 In collaboration with an interdisciplinary team of scientists from Germany and Sweden with combined expertise in physical organic, biological and theoretical chemistry as well as spectroscopy, they have now shown that the protein-bound astaxanthin is present as a negatively charged enolate ion that is blue in colour. This was demonstrated using a simplified model compound, 3-hydroxy-4-oxo-ß-ionone, chosen to replicate the deprotonation behaviour of astaxanthin while disentangling the results from other lesser effects arising from the rest of the larger astaxanthin molecule.
Enolate formation was actually considered by previous investigators in 1968,3 but discounted on the basis that crustacyanin did not have any sufficiently basic sites to deprotonate astaxanthin. Helliwell explains that while this is true, on the flip side of the coin the acidity of astaxanthin has to be properly considered. ‘On a more thoughtful examination of the functionality in astaxanthin, it should behave as an extended fat-soluble form of ascorbic acid [vitamin C]. Given that ascorbic is a strong organic acid, the acidity of astaxanthin seemed worth investigating. That is what we have done and discovered that it is indeed a relatively strong acid.’
‘This study presents a fascinating application of modern physical organic chemistry to a longstanding and enigmatic culinary observation,’ comments organic chemist Guy Lloyd-Jones from the University of Edinburgh, UK. ‘The outcome of the work is the beautifully simple, yet surprising, conclusion that the dark blue colour is a hydroxyenolate anion that is bathochromically shifted from the neutral a-hydroxy ketone.’
Helliwell asserts that the enolate anion of astaxanthin is the only viable explanation for the majority of the colour shift, but future work is planned to try and understand the different effects contributing to this phenomenon in finer detail.










No comments yet