While pharma companies stand accused of giving up on drugs to help people stop smoking, tobacco firms are looking at controversial nicotine replacement products. Anthony King surveys an area that is problematic on all sides.
While pharma companies stand accused of giving up on drugs to help people stop smoking, tobacco firms are looking at controversial nicotine replacement products. Anthony King surveys an area that is problematic on all sides.
But four out of five who try quitting on their own fail within the first month and just 3 to 5 per cent remain abstinent for six months. A recent review of drug options for tobacco dependence noted: ‘The pharmacological effect of nicotine plays a crucial role in tobacco addiction, and therefore pharmacotherapy is important to address this component of tobacco dependence in order to improve success rates.’1
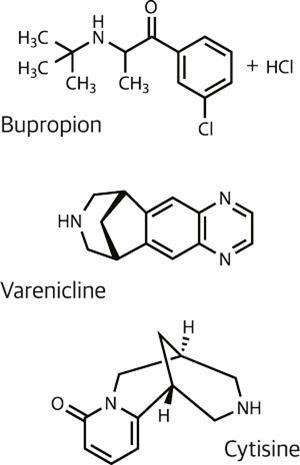
A global market of around 650 million potential customers is an alluring prospect for pharmaceutical companies, yet there are just three major options for drug-aided smoking cessation: various nicotine replacement therapies and the drugs bupropion and varenicline. Bupropion was discovered to improve smoking cessation when it was being used as an antidepressant; its mode of action is still unclear, but it has been sold as Zyban by GlaxoSmithKline. It is now off patent, and generics are available.
A Cochrane Review article found bupropion doubles the chances of quitting smoking compared with placebo, but the newer drug varenicline does better.2 Other research has shown that smokers had around a 2.5 greater chance of quitting using this drug compared with placebo, and about 1.7 times greater odds compared with bupropion.3,4 It works by stimulating the same receptor as nicotine (nicotinic acetylcholine receptors, nAChRs) and by blocking nicotine’s path to it.
Recent fallers
‘I suspect there is some degree of disappointment on the part of the pharmaceutical companies in how those medications, which have been approved, have done. Both in their effectiveness, but also their sales,’ says Thomas Glynn, a top tobacco expert at the American Cancer Society. In the US, Pfizer, Novartis and GSK remain involved in nicotine dependence products, but biotech companies appear to have given the market a wide berth. ‘I often wondered if a company like Genentech might get involved in this because I reckon that there must be other ways of looking at it,’ Glynn notes. More solutions would be welcomed: the estimated 6-month abstinence rate after receiving behavioural support is about 20% with pharmacotherapy and about 10% without .
There are several drugs under investigation that are believed to boost brain dopamine levels by stopping the metabolism of dopamine by monoamine oxidase type B (MAO-B). This improves transmission of dopamine and dampens down the dreaded nicotine withdrawal symptoms. The US National Institute on Drug Abuse (NIDA) looked at patch and oral formulations of the MAO-inhibitor selegiline in Phase II trials, but then the candidate hit the rocks. ‘We didn’t find any significant differences between selegiline and placebo in terms of rates of smoking or changes in cravings or any other outcomes,’ says Ivan Montoya, Deputy Director at NIDA’s Division of Pharmacotherapies and Medical Consequences of Drug Abuse
‘We basically abandoned that compound and that approach, although there is still science showing that nicotine dependence may be associated with changes in monoamines in the brain,’ says Montoya. ‘There is not much going on with this class of compounds now,’ says tobacco studies expert Robert West at University College London, who doesn’t foresee any work in this area in the near term.
Other classes have dropped toward the back of the field too. Nicotine binds to nAChRs and leads to the release of dopamine – which is involved in pleasure and reward mechanisms in the brain – and other neurotransmitters. The cannabinoid receptor system is thought to indirectly inhibit the dopamine reward buzzer, and antagonists of the cannabinoid receptor 1 were seen as promising. But these too drifted off course: Taranabant was discontinued by Merck & Co. in 2008 after a trial concluded that it did not improve smoking cessation. Merck currently has no publicly disclosed candidates for nicotine treatment.
A tricky shot
Vaccines are now many people’s favourites. They stimulate the immune system to produce antibodies against nicotine, heading it off before it reaches the blood–brain barrier. Nicotine is too small to be recognised by the immune system, so nicotine conjugated to a larger carrier protein is being tried. The trailblazer candidate was NicVAX, by Nabi Biopharmaceuticals/GSK, which had virus shells. Glynn sums up recent phase II trials: ‘Six months after treatment was completed, about 11% of smoking patients who used NicVAX had stopped smoking. Unfortunately the placebo was also about 11%, so they found no effect.’

It has proved challenging to produce a vaccine which mounts a complete blockade, explains West. ‘You have to come up with a conjugate which is going to fool the body into producing antibodies at a high enough rate and continue to do so for months after you have taken the vaccine,’ he says. If you could, you’d be on to a winner.
Compliance is an issue for existing cessation drugs, and this would likely be an issue for vaccines too. ‘It’s a fairly complicated regimen of injections for the vaccines, at least as they are planned right now,’ says Glynn. ‘And we already have trouble getting people to use nicotine patches, just putting one on every day.’
The hurdles
The major challenge now is not so much developing new treatments but getting people to use existing treatments more effectively, says West. Most smokers buy replacement therapy like nicotine gum or patches over the counter. ‘The evidence is that that is a less effective way of stopping smoking than going to the doctor and getting either nicotine replacement therapy or Champix or even Zyban on prescription, and even less effective if they were also to get behavioural support,’ says West.
West, who has worked with many pharma companies involved in tobacco replacement research, believes they need more behavioural research. He says about 1.5% of smokers each year go for the optimal treatment of drugs and behavioural support, when that should be 20–30%. ‘That gain is going to be achieved by getting more smokers to use the treatments that we know are most effective. That would be far more effective than anything we could possibly achieve by finding a more effective drug, realistically.’

Although not a new drug – it has been used in some eastern European countries for decades – the first modern trial of cytisine is a bright spot on the horizon. An alkaloid isolated from the seeds of an acacia (Cytisus laborinum), it is a partial agonist which binds with high affinity to the alpha4beta2 subtype of the nicotinic acetylcholine receptor. A trial published in 2006 reported a sustained 12-month quit rate of 8.4% in the cytisine group, compared with 2.4% for placebo. Cytisine would cost considerably less than other drugs, a big factor in countries like China and India, where replacement medication can cost far more than tobacco. ‘There is a good chance it [cytisine] will become the main smoking cessation drug, particularly in low income countries,’ says West, who worked on the 2006 study.5 But a cheap 40-year old drug is not going to gloss the pharma sector’s profit sheet.
Crisis and controversy
West is pessimistic about research and development on tobacco cessation drugs: ‘My impression is that [pharma companies] are not putting as much money into it as they have done.’ Montoya, however, remains optimistic that new drugs will come. NIDA has been working to encourage private partners to advance the development of new candidates, but this reflects a reduction in their interest.
‘The crisis is not just for nicotine. The crisis is in general for any neuropsychatric disorder,’ says Montoya. ‘Pharma companies are downsizing and some of them have closed their neuropsychiatric divisions because right now it is difficult to do that type of research and it is not economically profitable for pharmaceutical companies.’
Simon Chapman, professor of public health at the University of Sydney, takes a dim view of the ‘medicalization’ of quitting. The majority of those who have quit cigarettes have done so without drugs, and Chapman believes that at a population level drugs like bupropion have had no impact. ‘I don’t believe drugs ought to be promoted as the first line of effort in trying to get people to quit,’ he explains, adding that not all smokers should use drugs. He criticises a UK campaign for trying to suggest ‘that going cold turkey is not a sensible way to proceed, when we know from population evidence that it is.’
Those who say drugs won’t improve your chances of quitting ‘mislead the public,’ West counters, bristling. Whatever the tensions in this argument, they are mild compared to the controversy created by a new line of products from the tobacco industry.
Tobacco companies blur the lines
Reynolds American purchased Niconovum, a Swedish maker of gum and other nicotine replacement products, two years ago. Last year, Phillip Morris bought the rights to a new nicotine-infused aerosol technology that delivers nicotine directly to the lungs. And British American Tobacco announced a start-up, Nicoventures, which aims to provide an alternative to cigarettes, without the ‘real and serious health risks of smoking.’ The industry says it is engaged in harm reduction.
‘We have been trying for some time to see [...] whether people would substitute cigarettes for smokeless products. There are barriers and a key barrier is the habits people have,’ says Christopher Proctor, chief scientist at British American Tobacco. ‘There’s a whole bunch of rituals and other sensations that you don’t necessarily get with a pure nicotine product.’ Proctor says.
‘The company has to consider how to work with governments and with consumers to offer things which are much safer than cigarettes,’ Proctor says. ‘Cigarettes are clearly the most harmful way of using tobacco.’ But such moves have not impressed everyone.
Like many public health experts, Glynn believes you would not see wholesale switching to less harmful nicotine products; instead, people would continue smoking but use other nicotine products in places where smoking is banned, such as theatres and airplanes. ‘That would actually retard cessation by allowing people to continue to smoke,’ Glynn concludes.
Nicotine replacement solutions do not offer as quick a hit as lit tobacco. Cigarettes deliver nicotine quickly to the lungs as they ride on tar particles: nicotine is quite astringent but the harmful tar component makes it easier to inhale. Vapour products, on the other hand, do a poor job as the vapour coalesces in the mouth, where nicotine absorption is slower. ‘You’ve got to find a way of carrying nicotine on a particle that is going to get it into the lungs,’ says West, but one which is not harmful. But nicotine is not the only substance of interest.
The tobacco industry has reason to look more closely at flavourings, because the FDA could mandate nicotine to be reduced in cigarettes to a non-addictive level over a ten-year period, notes Glynn. Those other components of tobacco which would help maintain dependence or sell more products would become even more important.
‘We want to reduce the public health impact of smoking,’ Proctor says, ‘and we would expect that the marketplace would evolve from cigarettes to a range of other products that are a lot safer.’ He describes reservations by ‘some in the public health community’ as ‘understandable.’ But he adds: ‘There are people in the public health community who are quite frustrated by the lack of innovation in the pharmaceutical products. They haven’t been hugely effective for being a substitute for cigarette smoking. That’s partly because that wasn’t their intent, as medical products.’
Whether tobacco companies can ever be part of the solution, trust is not in ready supply from many people. ‘It [tobacco use] is an unequalled risk to the health of the developing world and increasingly the developed world,’ argues Michael Fiore of the University of Wisconsin Center for Tobacco Research and Intervention. ‘The fact that the tobacco industry makes a product which, when used as directed, kills people – and they have a history of lying and fraud, which dates many decades – means they cannot be trusted and cannot be part of the solution.’
References
1 R Polosa & N L Benowitz, Trends Pharmacol. Sci., 2011, 32, 281 (DOI: 10.1016/j.tips.2010.12.008)
2 L F Stead et al, Cochrane Database Syst. Rev., 2008, CD000146 (DOI: 10.1002/14651858.CD000146.pub3)
3 D E Jorenby et al, J. Am. Med. Assoc, 2006, 296, 56 (DOI: 10.1001/jama.296.1.56)
4 D Gonzales et al, J. Am. Med. Assoc., 2006, 296, 47 (DOI: 10.1001/jama.296.1.47)
5 R West et al, N. Engl. J. Med. 2011, 365, 1193 (DOI: 10.1056/NEJMoa1102035)











No comments yet