Attaching amino acids to a hydrophobic organic molecule allows them to self-assemble into rare, knotted structures with defined handedness, sidestepping the need for external templates or chiral resolution. The approach delivers chiral, mechanically interlocked molecules in a single step, opening new possibilities for molecular recognition, sensing and asymmetric catalysis.
Producing large interlocked molecules in a single mirror-image form is a long-standing challenge in chemistry. Chirality underpins everything from drug activity to biological recognition, yet controlling it becomes increasingly difficult as molecular architectures grow more complex. This is especially true for mechanically interlocked molecules, such as catenanes, knots and links, that are held together by their topology rather than by covalent bonds.
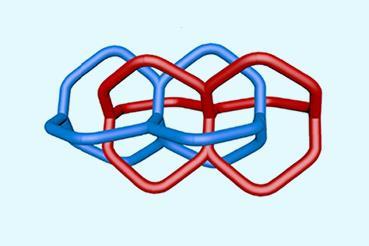
Solomon links are among the most intricate examples. Comprising two double interlocked rings with four crossing points, they are inherently chiral because of the way the strands cross. While few synthetic routes to these structures exist, they typically rely on predesigned templates that offer limited control over handedness and function.
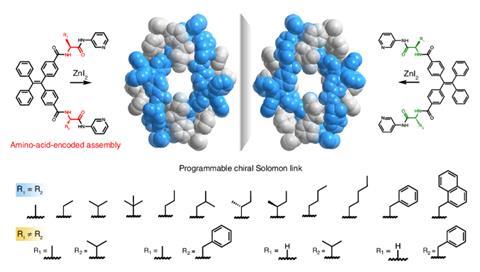
Now, a team led by Yong Cui and Jinqiao Dong at Shanghai Jiao Tong University, China, together with Anthony Davis at the University of Bristol, UK, has demonstrated a biologically inspired strategy that uses amino acids to programme tetraphenylethylene molecules into spontaneously forming chiral Solomon links.
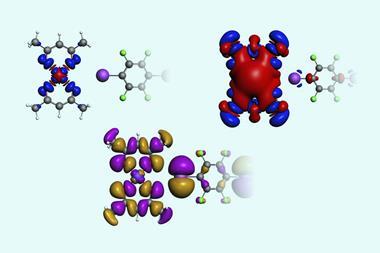
The researchers synthesised molecular building blocks comprising a rigid aromatic core flanked by amino acids with pyridyl groups that bind zinc ions. When mixed with zinc salts, the components self-assemble into Solomon links – but only when the amino acid residues share the same chirality. Networks of hydrogen bonds between the amino acids align the molecular strands precisely, determining which strand passes over or under another at key points, fixing the crossings needed to generate the knotted topology. Metal coordination then closes the rings, mechanically locking the structure in place. By contrast, achiral components or mixtures of left- and right-handed amino acids form simple, unentangled coordination polymers. ‘It is remarkable how different structures could be obtained through modulation of the enantiopurity of the ligands used,’ comments Jamie Lewis, from the University of Birmingham, UK, who was not involved in the work.
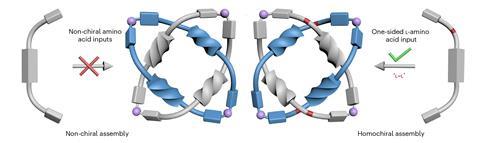
X-ray crystallography confirmed the formation of the doubly interlocked topology and revealed multiple layers of chirality, from the amino acid stereocentres to the overall molecular topology. Changing the amino acid side chains allowed the researchers to tune the size and chemical environment of the cavity formed at the centre of the Solomon link, which in turn affects function. The link selectively binds short peptides and can discriminate between enantiomers. When incorporated into polymer membranes, they can even detect biologically relevant targets, including the inflammatory biomarker interleukin-6, at nanomolar concentrations.
The researchers write that this amino-acid-driven approach offers a simple, one-step route to complex, chiral interlocked molecules. ‘Although it may not be trivial to generalise this approach to other classes of mechanically interlocked molecules, there is no inherent reason why this wouldn’t be successful,’ says Lewis. ‘Gaining detailed understanding of the interactions between fragments that allow transfer of chiral information will be critical to designing alternative systems using this approach.’
References
S-L Yan et al, Nat. Synth., 2026, DOI: 10.1038/s44160-025-00954-w













No comments yet