We take a look back at the year’s most interesting chemical science stories
What discoveries caused the biggest buzz in chemistry labs in 2013? With the help of an expert panel of journal editors Chemistry World reviews the ground breaking research and important trends in this year’s crop of chemical science papers.
Playing with DNA
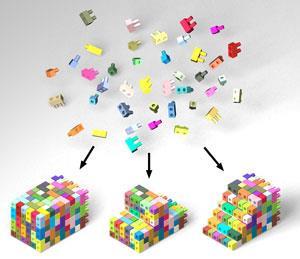
As ever, this year saw plenty of groups pushing the frontiers of DNA nanotechnology. In the US, Peng Yin and colleagues at Harvard University made nanoscale DNA bricks that could lock together like lego blocks (video) and be built into a huge range of different shapes.1 Each brick consists of a 32-nucleotide strand of DNA which has two ‘head’ domains – which protrude like the knobbles on lego bricks – and two ‘tail’ domains that the knobbles can slot in to. To build specific shapes, a computer is used to map out exactly which bricks are needed, then they are simply mixed so that they self-assemble. Although similar DNA origami methods have been used before to create three-dimensional structures, the modular building-block approach is a new twist that could prove useful to nanotechnologists in future.
Meanwhile, Giulio Malucelli and colleagues at the Polytechnic University of Turin in Italy used DNA extracted from herring sperm to make a fireproof coating for textiles.2 They discovered that DNA acts in a similar way to traditional flame retardants when exposed to fire: phosphoric acid from the phosphate backbone dehydrates the deoxyribose units to provide carbon, which acts as a thermal insulator, and nitrogen-containing bases release non-combustible ammonia gas which forms a physical barrier. The group suggested their DNA-based coating could be used as a safer, greener alternative to current flame retardant materials, but have yet to work out how to get it to survive the laundry process.
But back to one of DNA’s more conventional uses. For billions of years DNA’s four-letter code has acted as an information store for cells. Now a UK team led by Nick Goldman at the European Bioinformatics Institute have showed that it could be used to store other kinds of data, including one of Shakespeare’s sonnets and an audio recording of Martin Luther King’s ‘I have a dream’ speech.3 The group converted the original data into binary, and then used various information theory algorithms to translate each byte into a DNA sequence. Dried DNA is much longer lived and information dense than the hard disks we currently use – perhaps the future of data storage lies in DNA and other polymers.
Terminator polymer: I’ll be back
Scientists in Spain recently announced the first self-healing polymer that can repair itself without external intervention. After being cut in half, the material seamlessly heals itself at room temperature in just two hours.4 Ibon Odriozola and his group at the at the CIDETEC Centre for Electrochemical Technologies have dubbed their creation a ‘terminator’ polymer – a tribute to the shape-shifting, molten T-1000 robot from Terminator 2.
Self-healing polymers – that mend themselves by reforming broken cross-linking bonds – have been made before, but they usually require catalysts or specific environmental conditions to provide the energy for bond repair. Some of the group’s previous efforts – silicone elastomers that use silver nanoparticles as cross-linkers – have come close to spontaneous healing, but still required external pressure.5 Now, though, they seem to have found the key – the metathesis reaction of aromatic disulfides, which naturally exchange at room temperature, enable the bonds in their poly(urea–urethane) network to regenerate, making it perhaps the first truly self-healing polymer.
The new material is simple and inexpensive to produce, and the team say it would be ideal for commercial applications, to toughen up plastic parts in anything from electronics to biomaterials.
Crime scene chemistry
Improvements in forensic techniques have also featured on our pages this year. A team in the US developed a device that investigators can wear on their fingertips to rapidly identify traces of explosives and gunshot residue.6 The sensor consists of an electrode screen-printed onto a stretchable sheath worn on the index finder, and a sheath for the thumb coated with a solid-state ionogel electrolyte. To analyse a sample the investigator simply squeezes their finger and thumb together after swiping a surface, completing the electrochemical cell. A portable analyser then reads the voltammetric signal, identifying distinct peaks for explosives or gunshot residue. The process takes just a few minutes, cutting down the lengthy practice of sample collection and lab analysis.
Another group has developed a bioassay that can be used to analyse blood samples on-site to give investigators an early indication of a suspect’s ethnicity.7Evgeny Katz at Clarkson University, US, in collaboration with Jan Halámek, now at the State University of New York at Albany, analysed levels of two biomarkers – creatine kinase and lactate dehydrogenase – in the blood of people of Caucasian and African American ethnicity. They then developed a bioassay to amplify the differences in these levels. The test could successfully distinguish between ethnicities in real human blood samples, as well as samples a day old, as could well be the case at a crime scene.

Moving on to cold cases, the investigation into the death of former Palestinian leader Yasser Arafat has been making headlines throughout the year. His body was exhumed in 2012 for Swiss, French and Russian scientists to investigate claims that he was poisoned, after polonium-210 – was found on some of his clothes and possessions. But so far it has proved difficult to find a definitive answer, as so many radioactive half-lives of the radionuclide have passed since his death in 2004.
The Swiss team say their results moderately support the idea that he died from radioactive poisoning, as bone and tissue samples from the grave seem to contain higher levels of polonium-210 than would be expected from naturally occurring background sources. But the French team, by contrast, have concluded that Arafat did not die from poisoning but from natural causes. It seems that the question is now unlikely to ever be definitively answered by forensic investigation alone.
Hydrogen bonding under the microscope
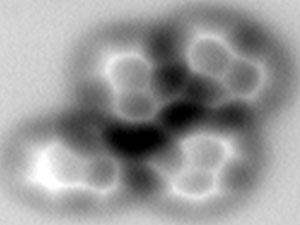
When researchers in China reported the first high quality visualisation of a hydrogen bond it caused quite a stir. While anyone who learned the basics of chemistry at school will be familiar with hydrogen bonds, actually seeing them was amazing. Xiaohui Qiu and colleagues at the National Centre for Nanoscience and Technology in China used atomic force microscopy (AFM) to produce remarkable pictures of the hydrogen bonds that formed between molecules of 8-hydroxyquinoline.8 Interestingly the AFM images also reveal hydrogen bonds where the hydrogen that is part of the bond is joined to a carbon atom – rather than a highly electronegative element as is still commonly taught in schools. IUPAC proposed redefining the hydrogen bond in 2010.
This followed on from work earlier in the year from Felix Fischer ‘s group at the University of California, Berkeley in the US, which used AFM to image molecules before and after a chemical reaction, capturing the formation of covalent bonds.9 Qiu and his group chose 8-hydroxyquinoline because the hydrogen bonds are out of plane with the flat ring structure, making it more visible. They hope that just like NMR and mass spectrometry, AFM will one day become a routine tool for examining molecules.
Driven by Curiosity
From murder mysteries to the mysteries of the wider universe, this year has seen some important results beamed back from the Mars Curiosity rover which landed on the red planet in August 2012. Equipped with a portable lab of analytical instruments, and a robotic arm for sample collection, the rover has been busily documenting Mars’s geology and atmospheric composition.

Curiosity was sent to try and answer the big questions, one of which is whether the planet could ever have supported life. Some of its early observations look promising. As well as carbon, hydrogen, oxygen, phosphorus and sulfur, the Sample Analysis at Mars (SAM) portable laboratory on board Curiosity has detected chlorinated hydrocarbons in soil samples from the surface of Mars, which point to the presence of complex organic molecules. When soil from the Rocknest site was heated in an oven, it released chloromethanes along with other volatiles, suggesting that chlorine is reacting with organic compounds. Of course, no one can rule out the possibility that the carbon comes from contaminants on the equipment brought from Earth, and the lack of definitive evidence of organics at the site is not entirely surprising given the surface’s exposure to UV radiation, cosmic rays and oxidants. Nasa scientists hope to have more luck with samples drilled from beneath the surface of older terrain.
Later in the year, in an area of Mars known as Yellowknife Bay – thought to be the dried up bed of an ancient lake – chemical analysis of a rock sample showed conditions on the planet’s surface may have once been more hospitable to life. Around 20% of the rock was clay minerals – a product of the reaction of igneous minerals with relatively neutral and unsalty water. The presence of calcium sulfate suggests that the surrounding soil was neutral or mildly alkaline, and compounds in varying states of oxidation – for example both sulfates and sulfides – were found, suggesting that the environment was not violently oxidising. Together these factors indicate the environment could have once supported ancient microbial life.
Nasa’s next Mars mission – the Maven orbiter which will investigate the upper atmosphere – has already blasted off.
Classic carbocation case closed
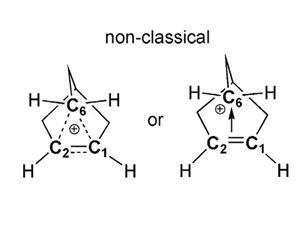
Earlier this year German scientists ended a long-running controversy over the 2-norbornyl carbocation, finally putting its nonclassical structure, with a pentacoordinate carbon, beyond doubt after more than 60 years.10
The debate began in 1949 when Saul Winstein first proposed nonclassical carbocations to explain the reactivity of substituted norborane compounds. But early opponents took issue with the idea that carbon might be bonded to more than four other atoms. 2-norbornyl carbocations became central to the dispute and work throughout next few decades, including NMR observations and molecular orbital calculations, favoured the nonclassical structure. Now, Karsten Meyer and colleages at Friedrich-Alexander-University in Erlangen have finally brought closure by solving the 2-norbornyl carbocation crystal structure.
The team had to follow a crystallography procedure that Mayer himself called ‘insane’. The sensitive 2-norbornyl cation crystals had to be handled at extremely low temperatures in a helium-cooled diffractometer and cooled down very gradually to prevent them cracking. After spending ‘a small fortune’ on helium, the work finally paid off and the team got the evidence needed to put the whole issue to rest.
Pringles and tubes
Back on Earth, the world of nanocarbon chemistry has taken some leaps forward. Chemists welcomed the newest member of the nanocarbon family when Kenchiro Itami’s group at Nagoya University in Japan, and colleagues in the US, created what can be thought of as a graphene ‘Pringle’.11 They made the warped, double-concave nanographene by embedding non-hexagonal rings into the graphene lattice, which caused the whole thing to bend. Being able to distort the structure in this way could make it possible to create tailor-made graphene structures for niche devices and applications in the not too distant future, and more warped graphene shapes are no doubt on the horizon.
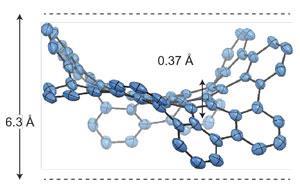
Itami’s group also developed a way to control the diameter of carbon nanotubes (CNTs) – something that has held back their widespread use in electronics – using cycloparaphenylene (CPP) nanorings as templates.12 Currently, the most common way of making CNTs – chemical vapour deposition – offers little control over their properties, producing a mixture of diameters and chiralities. Itami’s group coated CPP nanorings of set diameters onto tiny fragments of sapphires and exposed them to very hot ethanol vapour, and succeeded in growing CNTs with small diameters. Although the technique still needs honing, it offers huge potential for producing CNTs with the precision demanded by the electronics industry. The team predict that CNT production may eventually completely switch over to template-based methods.
In further CNT news, scientists at Tsinghua University in China smashed their own record for nanotube length when they grew a CNT that was over half a metre long.13 Their technique focused on optimising the conditions for catalyst activity, after they discovered its importance for the kinetics of CNT growth. Perhaps next year will see CNTs break the one metre mark.
In the US, researchers unveiled the first CNT computer, built entirely of CNT transistors.14Max Shulaker and colleagues at Stanford University were able to overcome some of the difficulties associated with making CNT chips, and created a prototype computer that uses a simple operating system to both count and sort numbers. It has long been predicted that CNT technology will one day overtake traditional silicon chips, which are reaching their limits in terms of miniaturisation. While there is still a long way to go, the development of a functional computer has shown this is a real possibility.
3D printed bionic ear
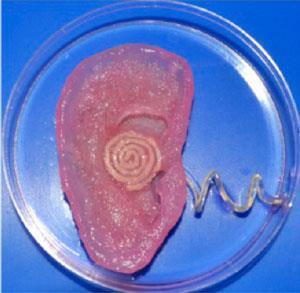
3D printing technology continues to go from strength to strength and one of this year’s greatest successes was this bionic ear made by Michael McAlpine’s group at Princeton University in the US. It is one of the first printed devices to integrate electronics with biological tissue (see the story for a video of the ear in action!).15
To make it, the group used three ‘inks’ in their printer: silicone infused with silver nanoparticles for the electronics; silicone alone for structural support; and finally a gel containing chondrocyte cells, which produce cartilage. Once the ear had been printed according to a three-dimensional blueprint fed into the printer, it was incubated in a culture medium to allow the cartilage to form.
The resulting ear certainly looks like the real thing – albeit without skin – and the coiled antenna at its centre functions in a similar way to existing cochlear implants. This work, and similar work from other groups, has put 3D printing well and truly on the radar as an approach to tissue engineering. We can’t help but wonder what the next body part to come out of a 3D printer will be.
Ancient history in the lab
And finally, this year has seen researchers delving into the past as well as shaping the future. The contents of ancient medicine cupboards were uncovered when researchers in Italy analysed some of the contents a doctor’s chest found in a 2000-year-old shipwreck.16 They took samples from large tablets, about 4cm wide by 1cm thick, found inside a tin container. Spectroscopy identified zinc as the most abundant element, present as smithsonite (zinc carbonate) and hydrozincite (zinc hydroxycarbonate). Going by ancient medicinal ‘recipes’ recorded by writers like Pliny the Elder and Dioscorides, it is likely that these zinc compounds were used to treat eye and skin conditions. Some of the compounds identified are still used in medicine today.
And it seems that some of our culinary traditions also have longer histories than we might expect, particularly our taste for spicy food. An international team found traces of the pungent herb garlic mustard on cooking pots used by European hunter gatherers around 6000 years ago.17 Distinctive fatty acids indicative of marine organisms were also found inside the pots, suggesting the spice was used to flavour fish and shellfish dishes.

Across the Atlantic, chemical analysis of the residues found inside 2000-year-old pottery from southern Mexico put the earliest use of chilli in cooking back hundreds of years.18 Traces of Capsicum plants inside spouted jars suggested the chillies were used in liquids – perhaps a hot spicy drink or a chilli-based sauce for flavouring food during ritual feasts.
And researchers continue to develop less destructive ways to look at historical artefacts. A group led by Graham Davis at Queen Mary University of London, UK, developed an x-ray imaging technique that could be used to read historical scrolls that are too fragile to unroll. The method uses high contrast x-ray microtomography to scan rolled up parchment and detect iron contained in iron gall ink – a commonly used ink in Europe between the 12th and 19th centuries. Specially designed software then produces a 3D computer model of the document which can be ‘unrolled’ virtually to reveal the text. The team used the technique to successfully scan the text inside a tightly wound 19th century scroll. They hope to develop interactive software that could help historians virtually unravel and study these otherwise unreadable documents.
On that note we bring an end to our roundup – as always, just a small snapshot of the last year’s research. We look forward to seeing what 2014 brings.
References
1 Y Ke et al, Science, 2012, 338, 1177 (DOI: 10.1126/science.1227268)
2 J Alongi et al, J. Mater. Chem. A, 2013, DOI: 10.1039/C3TA00107E
3 N Goldman et al, Nature, 2013, DOI: 10.1038/nature11875
4 A Rekondo et al, Mater. Horiz., 2014, DOI: 10.1039/c3mh00061c
5 R Martín, Chem. Commun., 2012, 48, 8255 (DOI: 10.1039/c2cc32030d)
6 A J Bandodkaret al,Analyst, 2013, DOI: 10.1039/c3an01179h
7 F Krameret al,Analyst, 2013, DOI: 10.1039/c3an01062g
8 J Zhang et al, Science, 2013. DOI: 10.1126/science.1242603
9 D G de Oteyzaet al,Science, 2013, DOI: 10.1126/science.1238187
10 F Scholzet al,Science,2013, DOI: 10.1126/science.1238849
11 K Kawasumiet al,Nat. Chem., 2013, DOI: 10.1038/nchem.1704
12 H Omachi et al, Nat Chem, 2013, DOI: 10.1038/nchem.1655
13 R Zhang et al, ACS Nano, 2013, DOI: 10.1021/nn401995z
14 M M Shulaker et al, Nature, 2013, 501, 526 (DOI: 10.1038/nature12502)
15 M S Mannooret al,Nano Lett., 2013, DOI: 10.1021/nl4007744
16 G Giachi et al, Proc. Natl. Acad. Sci., USA, 2013, DOI: 10.1073/pnas.1216776110
17 H Saul et al, PLoS One, 2013, DOI: 10.1371/journal.pone.0070583
18 T Powis et al, PLoS One, 2013, DOI: 10.1371/journal.pone.0079013












No comments yet