We take a look back at some highlights from last year’s chemical science research
It’s fair to say 2016 was an exciting year for chemistry. We had barely packed away last year’s Christmas decorations when Iupac rocked the world by confirming the discovery of four new chemical elements, completing the seventh row of the periodic table. Elements 113, 115, 117 and 118 have been named nihonium (Nh), moscovium (Mc), tennessine (Ts) and oganesson (Og) respectively, in recognition of the institutes and teams in Japan, the US and Russia who discovered them.
You can read more about how they were discovered, and what Yuri Oganessian had to say about the future of the periodic table in our in-depth feature.
Molecular machines
Another exciting moment for chemists was the announcement of this year’s Nobel prize, which went to Jean-Pierre Sauvage, Fraser Stoddart and Ben Feringa for their work on molecular machines. By building the first mechanically interlocking molecules into moving components like motors and switches, the trio laid the foundations for what is now a burgeoning field. Researchers today continue to build on their work, and last year saw some exciting developments.
David Leigh’s group at the University of Manchester in the UK developed a ‘robot’ molecule that can pick up molecular cargo, then move it to a new location before releasing it1. The robot is made up of a platform attached to a rotating arm, which has a thiol group at the gripping end. The arm can be made to bond to the 3-mercaptopropanehydrazide cargo and swing it from one end of the platform to the other by changing the pH conditions. This precise kind of action is unprecedented on such a miniscule scale and the team are hopeful that it will lead to further advances in molecular robotics.
Leigh’s group also created a molecular system of interlinked chains that mimics the way the chemical fuel adenosine triphosphate (ATP) powers motor proteins within living cells. The arrangement consists of a catenane where one small ring continuously rotates clockwise around a larger one by interacting with a chemical fuel, 9-fluorenylmethoxycarbonyl chloride.2
Meanwhile, researchers have managed to recreate the action of another crucial component of living cells – the ribosome. Andrew Turberfield’s group at the University of Oxford, UK – together with Rachel O’Reilly’s group at the University of Warwick – made a system of hairpin-shaped DNA strands that could assemble chains of peptide building blocks by binding to them and self-assembling into a pre-programmed DNA sequence.3 They say systems like these could one day help to power and control molecular robots or factories.
The robot octopus
Who doesn’t love a soft robot? The tentacle-twitching 3D printed robot octopus made by researchers at Harvard University in the US caught our eye earlier this year. Although the ‘octobot’ has few practical uses so far, it is an important proof of concept, being the first self-powered, non-tethered robot to be made entirely out of soft components.21 The bot doesn’t need to be connected to an external power source, nor does it include any internal batteries. Instead, it runs entirely on hydrogen peroxide, which breaks down in the presence of a platinum catalyst to produce gases that power its movements.
Synthetic chemists
Others focused on an entirely different kind of machine, exploring how computers could shake up the way we do chemistry.
Last year we learned about new software developed by Bartosz Grzybowski from the Ulsan National Institute of Science and Technology, South Korea, and the Polish Academy of Sciences, which could help researchers plan new syntheses in a fraction of the time it normally takes, without having to sift through piles of literature. Chematica maps out more than 10 million substances and the reactions between them. Users can enter a ‘destination’ molecule and the system will work out the best potential ‘pathways’, based on cost, substrate availability and number of steps, in a matter of seconds.4 Syntaurus, another piece of the group’s software, uses more than 20,000 chemical rules that have been manually encoded to come up with new syntheses. Its creators recently used it to map out a total synthesis for epicolactone, a complex natural product.5
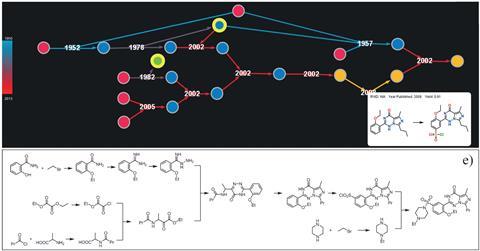
Meanwhile, other groups are using machine learning to predict the optimum conditions for reactions. A team led by Nishanth Chemmangattuvalappil and colleagues from the University of Nottingham Malaysia Campus developed a computational method that both designs carbon-capturing ionic liquids for carbon capture, and also predicts their prime operating temperature and pressure, the ease and cost of their synthesis, and their toxicity and corrosiveness.6
And Ichigaku Takigawa’s group at Hokkaido University in Japan has developed a method that reduces the time it takes to predict how well different metals will perform as catalysts.7
Stephen Buchwald and Klavs Jensen from the Massachusetts Institute of Technology, US, have gone further, combining machine learning approaches with practical chemistry to create a ‘robot chemist’: an automated flow reactor that can learn from its mistakes, optimising the conditions for catalytic reactions based on the results of previous experiments.8
The flat family
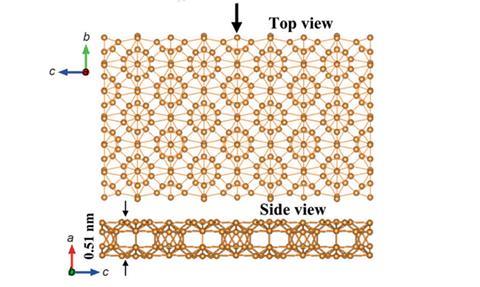
Graphene has some new friends in the land of 2D materials. In 2016 the first two-dimensional form of boron22 was synthesised. We also welcomed antimonene23 – a monolayer of antimony – that, unusually for a 2D material, was stable in water. Meanwhile, theoretical scientists predicted the existence of a new siligraphene24, SiC7, which the team believes could have solar cell applications if it can be made. On the flipside, the possibility that penta-graphene – a two-dimensional carbon allotrope made of pentagons – will ever be synthesised was ruled out using simulations by a group led by Christopher Ewels from the University of Nantes, France.25
A catalyst for change
Speaking of catalytic reactions, 2016 saw a number of advances in this arena.
George Olah and Surya Prakash’s team at the University of Southern California, US, developed a catalytic system that can make methanol straight from the tiny concentration of carbon dioxide found in Earth’s atmosphere – an important first step towards ‘air capture’ of atmospheric CO2. Their method involves bubbling air through a solution containing adsorbents that can capture CO2, and then bringing it together with a ruthenium catalyst and hydrogen, which results in the formation of methanol.9 The group hope that if the process can be scaled up, it could allow fuels or other useful products to be produced directly from air-captured carbon dioxide.
Another new catalyst is allowing drugs to be labelled with the radioactive hydrogen isotope tritium in a way that minimises radioactive waste. Unlike the iridium or rhodium catalysts that are often used for this purpose, the iron catalyst developed by Paul Chirik’s lab at Princeton University, US, and researchers at Merck, functions at low pressures of tritium. It also enables molecular sites that were previously inaccessible to be labelled, which could offer new ways to test the efficacy and safety of drugs.10
And when it comes to the search for new catalysts, there have been successes for low-tech approaches too. We reported a few months back that a team led by Remzi Becer at Queen Mary University of London, UK, had used a one-penny coin in place of copper wire to catalyse polymerisation. The coin was found to actually work faster than wire, and a far more cost-effective option with current copper prices. The penny can even be spent afterwards.11
Elsewhere, a group at the University of Kragujevac in Serbia showed that lemon juice can act as a cheap, green alternative to metal catalysts in the preparation of bioactive molecules. Nenad Janković and colleagues were able to make quinoxalines and benzoxazines from substituted ethyl 4-oxo-2-butenoates and ortho-phenylenediamine or ortho-aminophenol with excellent yields, with the citric acid in lemon juice acting as both a solvent and a biocatalyst.12
Flipping reaction
Images produced using atomic force microscopy (AFM) have dazzled us in recent years. In 2016, researchers continued to use the technique to probe chemical reactions in exquisite detail. Leo Gross and his co-workers at the IBM Research Centre in Switzerland were able to switch a single molecule reaction back and forth using voltage pulses applied to the needle tip of a scanning tunnelling microscope, inspecting their results using AFM.26 First, they used two pulses to break open a carbon–carbon bond in a molecule containing three aromatic rings, converting two of the rings into a larger one. With another pulse, they could ‘pinch’ the large ring back into two.
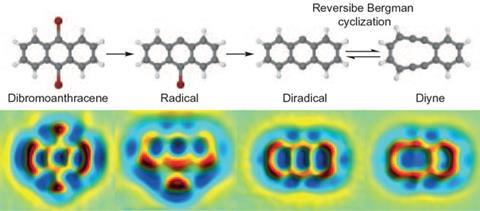
Printing possibilities
Over the past few years 3D printing has begun to open up new possibilities to chemists working across a range of disciplines, from materials design to tissue engineering. The technology continued to move forward in 2016.
Anthony Atala’s group at Wake Forest Institute for Regenerative Medicine in North Carolina, US, came closer than ever before to printing tissues and organs for medical transplants, by developing a 3D printing system that can build large areas of cells that stay nourished and maintain their structural integrity. Their device prints a soft, nutrient-rich hydrogel containing living cells alongside a biodegradable polymer ‘mould’ that holds the tissue together at first, but later disappears. The printer can also incorporate microchannels into the structures to provide space for oxygen and nutrients to be fed to the tissues, much like a system of blood vessels in actual organs. The team showed they could print three different tissue types – bone, muscle and a human-scale ear made of cartilage – that continue to survive and develop several weeks after being transplanted into rats or mice.13

Another 3D printing first was achieved by Tobias Schaedler and colleagues at HRL laboratories in the US, who were able to print ceramic structures. Because they are so brittle, ceramic materials usually have to be made by sintering – fusing powder grains at very high temperatures. To get around this, Schaedler’s group printed microlattices out of pre-ceramic polymers that pyrolyse into ceramics when heated. As the polymers can be deposited as high-purity liquids, the lattices have fewer flaws than ceramics manufactured by conventional processes, making them much stronger. The potential applications range from micro-electromechanical systems to jet engines.14
Meanwhile, A group at the University of Miami, US, led by Adam Braunschweig have added a new dimension to the technique and developed a process they call 4D printing, which controls the chemical composition of printed polymer patterns as well as their three-dimensional position (CW June, p26).15 Their system can print patterns of different brush polymers close together on a glass surface, independently controlling their position at sub-micrometre resolution using arrays of printing tips attached to microfluidic cells. Different monomers can be introduced into the cells to alter the chemical composition of the polymers. The team says this approach is taking them closer to being able to replicate both the architectural and chemical complexity seen in biological materials.
Goodbye greys
As 2016 drew to a close, we were wowed by the first ever colour electron microscope images, made possible by work from Mark Ellisman’s and Roger Tsien’s groups at the University of California, San Diego in the US.27 The technique involves encoding photosensitive versions of the proteins into the DNA of the cells to be imaged, which are then bathed in diaminobenzidine (DAB) monomers that contain a rare earth element such as lanthanum. When the photosensitive proteins are exposed to a specific wavelength of light, they release reactive oxygen species, which hit any DAB monomer within 5nm and cause it to polymerise. The distribution of the rare earths bound to polymerised DAB can then be mapped using energy-loss spectra, which is distinctive for each element, and colour is applied to mark their location. The technique was used to track protein dynamics between interacting nerve cells.
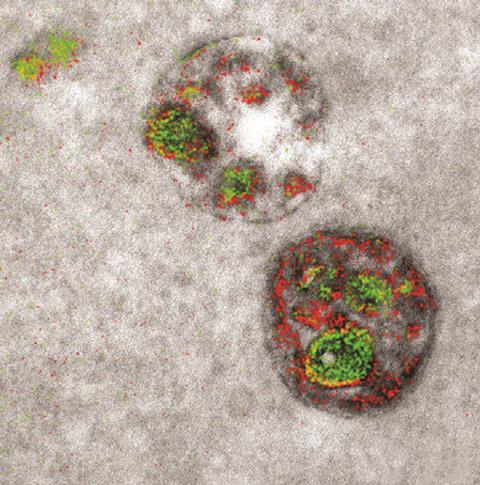
When the sun shines
The sun also shone on solar technology in 2016, with a number of new advances reported.
Perovskite solar cells are currently the hot ticket item. Midway through last year the Swiss team led by Michael Grätzel from the Swiss Federal Institute of Technology in Lausanne smashed their own efficiency record for a 1cm2 perovskite solar cell, achieving a certified efficiency of 19.6% by making improvements to the manufacturing process.16
But perovskite technology is still plagued by stability issues – there are still very few verified measures of efficiency simply because many of the cells degrade too quickly on exposure to air or sunlight. And new work by Robert Palgrave and his team at University College London, UK, has cast doubt on the chance of finding new perovskites to build solar materials that avoid this problem. The group showed that some of the calculations used to make predictions about the stability of undiscovered perovskites are flawed, and may produce results that will only lead to disappointment when syntheses are attempted further down the line.17 Other groups remain undeterred, however, and the hunt for new solar materials goes on.
When it comes to other solar cell technologies, researchers have continued to work towards improvements. Qunwei Tang and his colleagues at the Ocean University of China developed ‘all weather’ solar cells that can generate electricity using both sunlight and rainfall – a particularly attractive prospect for rainy countries such as the UK. The dye sensitised solar cell (DSSC) exploits ionic charge in salty raindrops to generate current.18 The same group also made a cylindrical DSSC that generates stable electrical output regardless of the angle of the sun.19

And Vladimir Bulovic, Annie Wang and Joel Jean from the Massachusetts Institute of Technology unveiled the lightest ever solar cell – so thin, it can sit on the surface of a soap bubble.20 The cell, which is 1.3µm thick and weigh sjust 3.6g/m2, was produced using vapour deposition to manufacture the supportive substrate, a protective overcoat and the organic light-absorbing components in a single process, rather than making each layer separately and then assembling them. The group thinks a similar approach could be used with other materials, perhaps even those tricky perovskites.
And on that sunny note we bring our round up of last year’s research news to a close. We can’t wait to see what 2017 brings.
References
1 S Kassem et al, Nat. Chem., 2015, DOI: 10.1038/nchem.2410
2 M R Wilson et al, Nature, 2016, DOI: 10.1038/nature18013
3 W Meng et al, Nat. Chem., 2016, DOI: 10.1038/nchem.2495
4 M Kowalik et al, Angew. Chem., Int. Ed., 2012, 51, 7928 (DOI: 10.1002/anie.201202209)
5 S Szymkuć et al, Angew. Chem., Int. Ed., 2016, 55, 5904 (DOI: 10.1002/anie.201506101)
6 F K Chong et al, Mol. Syst. Des. Eng., 2016, DOI: 10.1039/c5me00013k
7 I Takigawa et al, RSC Adv., 2016, 6, 52587 (DOI: 10.1039/c6ra04345c)
8 B J Reizman et al, React. Chem. Eng., 2016, DOI: 10.1039/C6RE00153J
9 J Kothandaraman et al, J. Am. Chem. Soc., 2015, DOI: 10.1021/jacs.5b12354
10 R Pony Yu et al, Nature, 2016, 529, 195 (DOI: 10.1038/nature16464)
11 R Aksakal, M Resmini and C R Becer, Polym. Chem., 2016, DOI: 10.1039/C6PY01295G
12 J Petronijević et al, Green Chem., 2016, DOI: 10.1039/c6gc02893d
13 H-W Kang et al, Nat. Biotechnol., 2016, DOI: 10.1038/nbt.3413
14 Z C Eckel et al, Science, 2016, 351, 58 (DOI: 10.1126/science.aad2688)
15 X Liu et al, Polym. Chem., 2016, DOI: 10.1039/c6py00283h
16 X Li et al, Science, 2016, DOI: 10.1126/science.aaf8060
17 W Travis et al, Chem. Sci., 2016, DOI: 10.1039/c5sc04845a
18 Q Tang et al, Angew. Chem., Int. Ed., 2016, 55, 1 (DOI: 10.1002/anie.2016608584)
19 Q Tang et al, Chem. Commun., 2016, DOI: 10.1039/c5cc10105k
20 J Jean, A Wang and V Bulovic, Org. Electron., 2016, DOI: 10.1016/j.orgel.2016.01.022
21 M Wehner et al, Nature, DOI: 10.1038/nature19100
22 G Tai et al, Angew. Chem., Int. Ed., 2015, DOI: 10.1002/anie.201509285
23 P Ares et al, Adv. Mater., 2016, 28, 6332 (DOI: 10.1002/adma.201602128)
24 H Dong et al, Nanoscale, 2016, DOI: 10.1039/c6nr00046k
25 C P Ewels et al, Proc. Natl. Acad. Sci. USA, 2015, DOI: 10.1073/pnas.1520402112
26 B Schuler et al, Nat. Chem., 2016, DOI: 10.1038/nchem.2438
27 S R Adams et al, Cell Chemical Biology, 2016, 23, 1, (DOI: 10.1016/j.chembiol.2016.10.006)












No comments yet