The tiny shear forces proteins experience inside blood vessels can dramatically increase their reaction rates, researchers have shown. The effect, which arises from changes in the proteins’ macromolecular shape, could even affect the action of therapeutic antibodies.
The forces on cells and biomolecules within living environments can affect their behaviour. Stem cells, for example, differentiate to become a specialised cell based partly on the elasticity of the surrounding extra-cellular matrix. Proteins crowded too tightly inside cells are more likely to convert into the amyloid fibrils associated with Alzheimer’s disease and Type II diabetes.
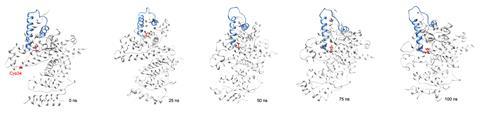
However, explanations for these phenomena have largely been restricted to the biological and chemical level – cell signalling and concentration. Subtle mechanical forces have rarely been considered. While the force-induced effects on reactions are being studied in the growing field of mechanochemistry, those forces are usually much larger than those in living systems – sometimes large enough to break bonds.
Researchers in the UK and Spain have now studied how the tiny shear forces – comparable to those exerted in the bloodstream by capillary walls – affect proteins reactions. ‘The proteins are locally deformed by these forces,’ explains Francisco Corzana of the University of La Rioja of what he discovered conducting molecular dynamics simulations. ‘So they probably retain their biological activity, but some amino acids that were masked are now exposed to the solvent and can react easily.’
The team also carried out microfluidics experiments at various pressures, targeting amino acids with reagents that become fluorescent on reaction. Their results matched the simulations, with biologically plausible shear stresses leading to increases in reaction rates by a factor of two in some cases. ‘It has previously largely been assumed that these modest levels of shear would have little effect on the chemical reactivity of the protein,’ says Tuomas Knowles of the University of Cambridge. ‘But our work shows that this is not necessarily the case.’
Studying trastuzumab, the monoclonal antibody developed to treat breast cancer, showed that ‘some of these antibodies may be partially dissociated under these shear stress conditions’, says Cambridge’s chemical biologist Gonçalo Bernardes. ‘There are many such antibodies now in the clinic and it’s important to understand how they behave, for example, while in circulation.’
‘This idea that you apply forces to proteins and whatever was inside now becomes exposed, and that this triggers reactivity of the previously cryptic sites is nice, but it’s already in the literature,’ comments physical biologist Sergi Garcia-Manyes of the Francis Crick Institute, UK. ‘What I think is new here is that you don’t have to completely unfold the protein – a small conformational change is enough to trigger some reactivity.’
Dennis Discher of the University of Pennsylvania, US, who has published on the mechanics of protein folding, notes that ‘every biochemistry textbook shows proteins changing conformation with temperature’. However, this refers to extreme temperature changes that don’t happen in living systems. ‘A lot of biochemistry in a test tube has been insightful but physiologically irrelevant,’ he says. ‘The shear stress effect is relevant – it’s happening in you with every beat of your heart.’
References
T A Hakala et al, J. Am. Chem. Soc., 2021, DOI: 10.1021/jacs.1c03681





![An image showing a [2]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/0/0/4/504004_fja0c01757_0007.jpeg_49119.jpg)










No comments yet