Ben Valsler
This week, Neil Withers examines the disproportionate effects of bonding in diborane…
Neil Withers
One of my favourite things about chemistry is that sometimes a little change in something’s composition results in a little change in its properties, but sometimes it results in something much bigger.
And a great example of that is diborane. As you can probably tell from the name, it’s got boron in it. Boron is the next door element to carbon, so you might expect compounds of say boron and hydrogen to be pretty similar to the many hydrocarbons we all know and love.
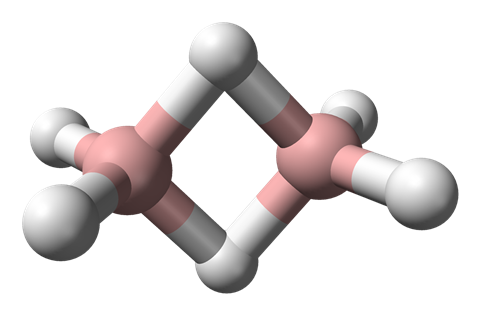
But you’d be wrong. Diborane has two boron atoms, and the rest is hydrogen. So you might think that it’d be like ethane with its two carbon atoms – but perhaps with only four hydrogen atoms to compensate for boron’s lower valency than carbon. But no – diborane is just like ethane, with six hydrogen atoms.
‘But boron only has 3 valence electrons!’ I hear you cry. ‘How on earth does that work?!’ This is where the fun – or rather the big differences in chemistry unleashed by small changes – begins.
Unlike narcissistic old carbon, boron is much less happy bonding with itself. So rather than two BH2 units forming a boron–boron bond to make a nice neat ethane-like B2H4 molecule, diborane does something different – something weird.
The two other hydrogen atoms each form a bridge between the two boron atoms, creating a B2H2 square ring. But as a square, as we all know, has 4 sides, so how does diborane use its 1 spare electron per boron atom, plus 1 per hydrogen atom, to make 4 bonds?
It cheats. It doesn’t make 4 bonds. Each boron–hydrogen–boron unit is joined by just two electrons. These are known as 3-centre-2-electron bonds. It’s pretty clear that the bonding in diborane isn’t straightforward when you look at its bond-lengths. The distance from the boron atoms to the non-bridging hydrogen atoms is much shorter than to the bridging ones, reflecting the weaker bonding in the bridging unit.
As a result, diborane is, unlike ethane, quite a reactive compound, making it a very versatile reagent in organic synthesis. It’s so reactive – and so light as boron is the fifth lightest element – that it was even investigated as a potential rocket fuel. You might think that in these reactions diborane was trying to grab a couple of extra electrons to become a dianion so it could bond more like ethane, which as we know is pretty stable. But that’s not the case: diborane has just as many electrons as it needs. The central B2H2 ring forms two bonding molecular orbitals, and those are filled by the four available electrons.

Admittedly, in many of its reactions diborane does react with electron donors so that each boron atom ends up with 8 valence electrons and can do more normal 2-centre-2-electron bonding, but it doesn’t form the dianion.
You can add more boron-hydrogen units to diborane to extend the series, a bit like alkanes. But again, the similarities end there. Instead of being simple linear or branched compounds, boranes tend to form polyhedral clusters, often with a single boron-hydrogen unit at the corner.
The moral of the story is perhaps not to view elements other than carbon as just ‘heteroatoms’ that will impart a little functionality on your nice organic molecule. It’s the differences between elements and their chemistry that is interesting and important, not their similarities. Perhaps the same could be said of people too.
Ben Valsler
Neil Withers with a thought for the week, and the interesting properties of diborane. Next week’s podcast will be our last before a Christmas break – and I’ll be bringing you one of the compounds responsible for the distinctive smell of the festive season, which is found in spices, dental pastes and bug repellents! If there are any compounds you would like us to tackle in the new year, please get in touch – email chemistryworld@rsc.org or tweet @chemistryworld. I’m Ben Valsler, thanks for joining me.












1 Reader's comment