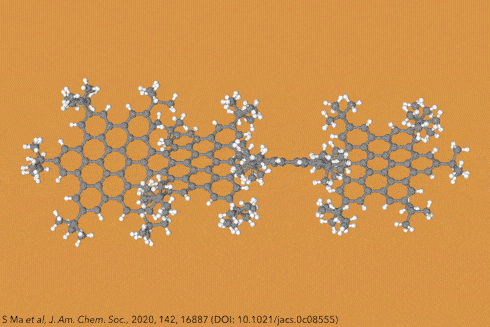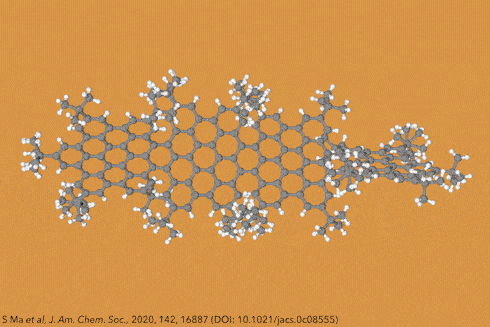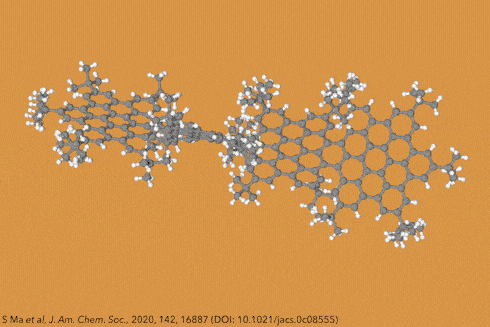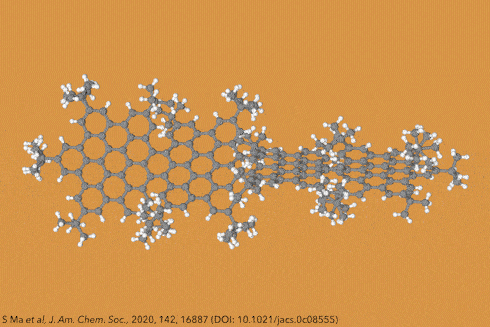
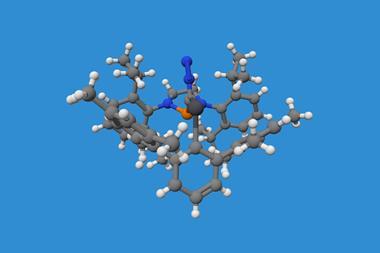
For the first time, chemists created dodecaphenyltetracene. It consists of a tetracene – four fused benzene rings – substituted on every possible position with more benzene rings, 12 of them in total.
Although there are longer and more twisted acenes, this one is the bulkiest, most sterically crowded acene ever made.
It was synthesised in only three steps by a team around Robert Pascal at Tulane University in New Orleans, US. Pascal and his colleagues are experienced acene-makers, having created shorter acenes – containing two and three central fused rings, respectively – more than two decades ago.
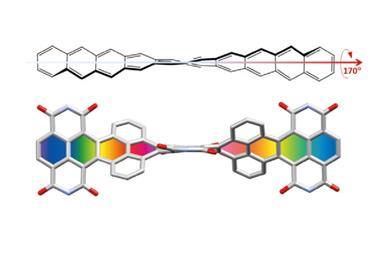
Their new perphenylacene is deep red and highly luminescent. The molecule is unusually unreactive and only slowly decomposes in air. Its 12 benzene substituents – which aren’t part of the acene’s aromatic system since they sit perpendicular to it – act like a picket fence. Like an inert hydrocarbon sheath, they encapsulate the acene, whose electronic structure remains essentially unaltered compared with plain tetracene.
Although dodecaphenyltetracene’s 97° end-to-end twist means it could theoretically be chiral, its enantiomers interconvert easily in a four-step procedure. The researchers estimate that each enantiomer has a half-life of only one second.
While not of immediate practical use, acenes have interesting optoelectronic properties that might make them useful in sensors or electronic components. Pentacene, for example, has been used as a semiconductor in organic electronics.
References
Y Xiao et al, Angew. Chem. Int. Ed., 2019, 58, 2831 (DOI: 10.1002/anie.201812418)


















No comments yet