Chemists toiling to synthesise organic molecules are set to have their tasks eased by a new carbon–carbon bond forming reaction, whose experimental procedure its inventors have described as ‘ridiculously simple’.1Phil Baran from Scripps Research Institute in San Diego, US, and colleagues bolt dialkylzinc reagents onto carboxylic acids at room temperature and pressure. That does an especially difficult chemical job – attaching two carbon atoms that each have three other single bonds, known as sp3 carbons.
The approach’s power is already simplifying drug research for Baran’s co-authors at the New Jersey laboratories of pharmaceutical company Bristol-Myers Squibb (BMS). ‘BMS is actively applying and further optimising this reaction in a number of programmes,’ Baran reveals. ‘This is pure translational chemistry: from invention to application in drug discovery in less than three months.’
Baran’s team had been focusing on carboxylic acids, one of the cheapest and most readily available compound classes around, with thousands of structures sold commercially. They’re heavily used in drug discovery research in reactions with amine molecules, giving amide products with diversity in shape and other properties that affect their biological activity. Amide formation requires a routine preparatory step turning the carboxylic acid into an active ester. Likewise, the new process begins from this common step.
Already this year, the Scripps and BMS researchers have published a process adding aromatic rings to carboxylic acids with ‘a high degree of practicality’.2 More specifically, it couples arylzinc reagents prepared from aryl bromides with active esters using a cheap nickel chloride catalyst and a bipyridine ligand. Though their method is done at room temperature, Baran’s team protects the reaction from atmospheric oxygen by filling vessels with argon. The reaction joins the sp3 carbon adjacent to the carboxylic acid group, which is eliminated as carbon dioxide, to the aromatic sp2 carbon adjacent to the zinc atom.
Zincing up a new route through chemical space
The chemists wondered if the approach could provide a straightforward path to other areas of chemical space by joining sp3 carbons, knowing this synthetic route is surrounded by pitfalls. ‘The issue with sp3 coupling is the myriad by-products one can envisage in such systems,’ Baran explains.
Exploring around a dozen alternatives to the bipyridine ligand soon provided optimised conditions that worked effectively with a simple test dialkylzinc and active ester pairing. The same conditions joined a surprisingly broad range of partners well, working with a wide variety of carboxylic acids and dialkylzinc reagents, containing several other potentially reactive structural elements.
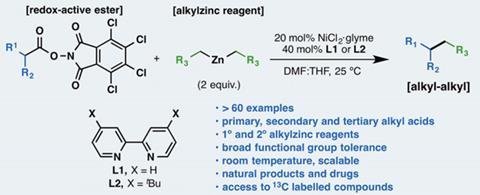
To demonstrate the method’s potential, the chemists applied it to structures involved in synthesising drugs or complex natural molecules. They suggest the approach’s most impressive feat is coupling dialkylzinc reagents to amino acid residues being chained together to make proteins while they’re attached to resin beads. And Baran stresses the straightforwardness of all these efforts. ‘If you have the skill to make an amide bond, you can make a carbon–carbon bond too,’ Baran says.
The main downside is that the approach doesn’t meet the high standards for ‘atom economy’ desired by green chemistry. The conditions call for twice as much dialkylzinc reagent as carboxylic acid, with the excess lost as by-product, which would be especially troubling if the zinc-derived fragment was valuable. Similarly, the carboxylic acid activating group is intentionally lost as a by-product. However, Baran emphasises that the activating reagent they use is cheap. ‘For decades people have made amide bonds in industry without much regard for atom economy,’ he adds. Furthermore, his team has ‘a laundry list of follow-up studies’ to improve and expand the method, with BMS, for example, looking for better bipyridine ligand alternatives.
Instant winner
‘I have no doubt that this chemistry will see wide use almost immediately,’ says Daniel Weix from the University of Rochester. ‘This work is a big advance for forming sp3–sp3 carbon–carbon bonds and a great example of the good that can come from industrial–academic collaborations. While the cross-coupling of alkylzinc reagents with alkyl halides has been known for some time, this work contains a surfeit of advances: the use of carboxylic acid derivatives instead of alkyl halides, the ability to form a wide variety of linkages and compatibility with a common solid phase.’
Anita Maguire from University College Cork, Ireland, underlines that application to solid phase peptide synthesis has clear potential in medicinal chemistry, allowing production of diverse compound libraries. ‘While the relatively low atom economy is acknowledged, the power of carbon–carbon coupling using readily-accessible and easily-handled carboxylic acids as precursors will undoubtedly render this an attractive methodology in the toolkit for organic synthesis,’ she says.
While Baran is hesitant to make predictions, he’s quietly confident the new reaction will prove useful. ‘This was a reaction that needed to be invented,’ he says. ‘If it’s only 1% as popular as amide bond formation it should have a significant impact in drug discovery.’
References
1 T Qin et al, Science, 2016, DOI: 10.1126/science.aaf6123
2 J Cornella et al, J. Am. Chem. Soc., 2016, 138, 2174 (DOI: 10.1021/jacs.6b00250)
















No comments yet